System A |8 J System B | 8 J 7J 6J Energy 4J 3J |2J 4J 2J Question 21 options: System A has more entropy because it has greater separation between energy levels and hence more microstates than system B. System B has more entropy because it has more than two mierostates, whereas System A only has two 654 3
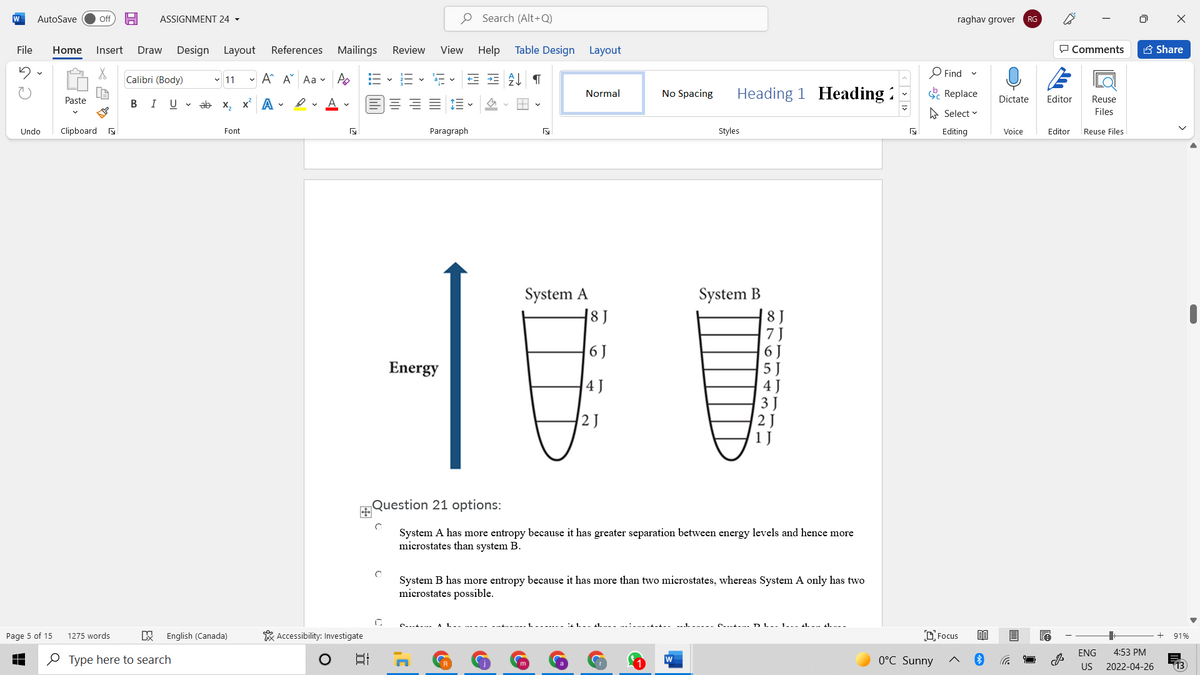
Consider the two different systems, A and B, shown below. If there are 3 particles with a total energy of 12 J, which system would have more entropy and why?
Question 21 options:
|
System A has more entropy because it has greater separation between energy levels and hence more microstates than system B. |
|
|
System B has more entropy because it has more than two microstates, whereas System A only has two microstates possible. |
|
|
System A has more entropy because it has three microstates, whereas System B has less than three microstates possible. |
|
|
System B has more entropy because it has more than three microstates, whereas System A only has three microstates possible. |
|
|
System A has more entropy because it has more than one microstate, whereas System B only has one microstate possible. |
Step by step
Solved in 2 steps with 1 images









