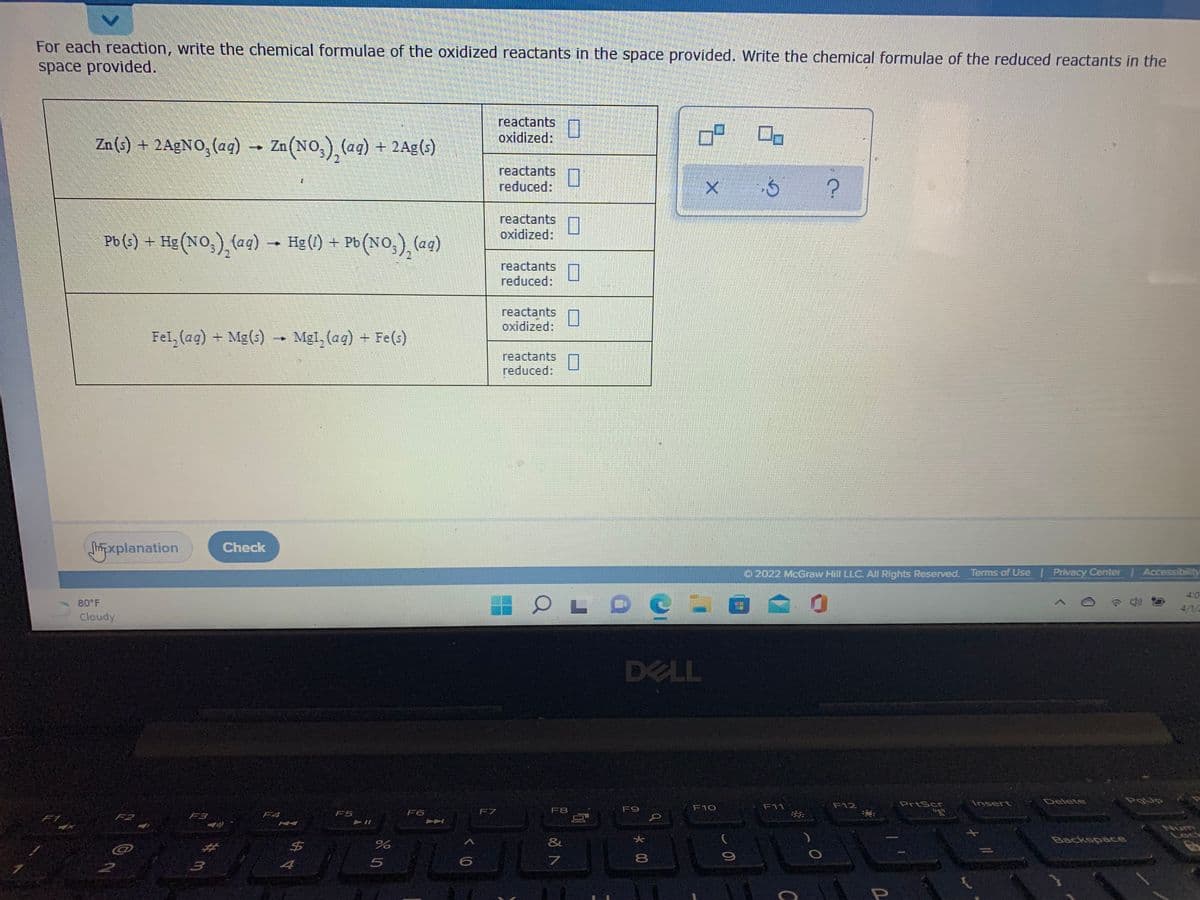
For each reaction, write the chemical formulae of the oxidized reactants in the space provided. Write the chemical formulae of the reduced reactants in the space provided. reactants oxidized: On Zn(3) + 2AGNO, (aq) - Zn(NO,), (aq) + 2Ag(s) reactants reduced: reactants oxidized: Pb (s) + Hg (NO,), (aq) - Hg (1) + - Po (NO.), (eg) reactants reduced: reactants oxidized: Fel, (ag) + Mg(s) - Mgl, (ag) + Fe(s) reactants reduced:
Science behind corrosion-test
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Corrosion
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images






