For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. Note for advanced students: you may assume ideal gas and ideal solution behaviour. System Change AS AS < 0 The seawater is passed through a reverse-osmosis filter, which O AS = 0 A liter of seawater at 15°C. separates it into 750. mL of pure O As > 0 water and 250. mL of brine (very salty water). not enough O information O AS < 0 20. L of pure helium (He) gas and 20.0 L of pure krypton (Kr) gas, O AS = 0 The gases are mixed, with the pressure kept constant at 3 atm. O As > 0 both at 3 atm and 7°C. not enough information O As < 0 1.0 g of ammonium chloride O AS = 0 (NH Cl) and 2.0 L of pure water The ammonium chloride is dissolved in the water. O As > 0 at 38 °C. not enough O information O O O O|0 O O O
For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. Note for advanced students: you may assume ideal gas and ideal solution behaviour. System Change AS AS < 0 The seawater is passed through a reverse-osmosis filter, which O AS = 0 A liter of seawater at 15°C. separates it into 750. mL of pure O As > 0 water and 250. mL of brine (very salty water). not enough O information O AS < 0 20. L of pure helium (He) gas and 20.0 L of pure krypton (Kr) gas, O AS = 0 The gases are mixed, with the pressure kept constant at 3 atm. O As > 0 both at 3 atm and 7°C. not enough information O As < 0 1.0 g of ammonium chloride O AS = 0 (NH Cl) and 2.0 L of pure water The ammonium chloride is dissolved in the water. O As > 0 at 38 °C. not enough O information O O O O|0 O O O
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter4: Gibbs Energy And Chemical Potential
Section: Chapter Questions
Problem 4.1E: List the sets of conditions that allow dS, dU, and dH of a process in a system act as a spontaneity...
Related questions
Question
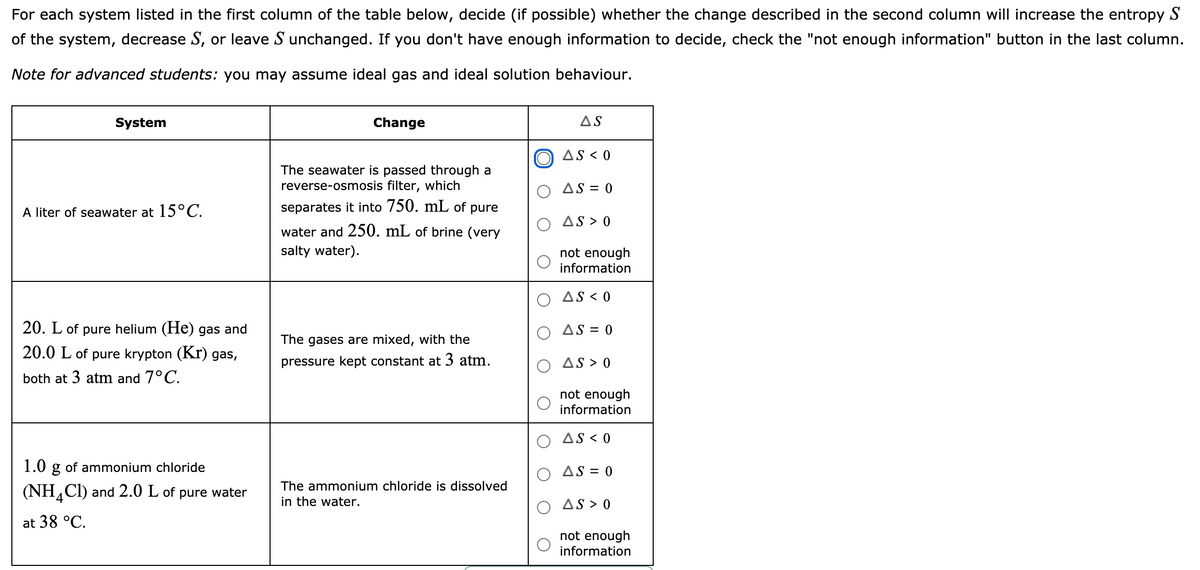
Transcribed Image Text:For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S
of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column.
Note for advanced students: you may assume ideal gas and ideal solution behaviour.
System
Change
AS
AS < 0
The seawater is passed through a
reverse-osmosis filter, which
AS = 0
A liter of seawater at 15°C.
separates it into 750. mL of pure
AS > 0
water and 250. mL of brine (very
salty water).
not enough
information
AS < 0
20. L of pure helium (He) gas and
AS = 0
The gases are mixed, with the
20.0 L of pure krypton (Kr) gas,
pressure kept constant at 3 atm.
AS > 0
both at 3 atm and 7°C.
not enough
information
AS < 0
1.0 g of ammonium chloride
AS = 0
(NH,CI) and 2.0 L of pure water
The ammonium chloride is dissolved
in the water.
4
AS > 0
at 38 °C.
not enough
information
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,