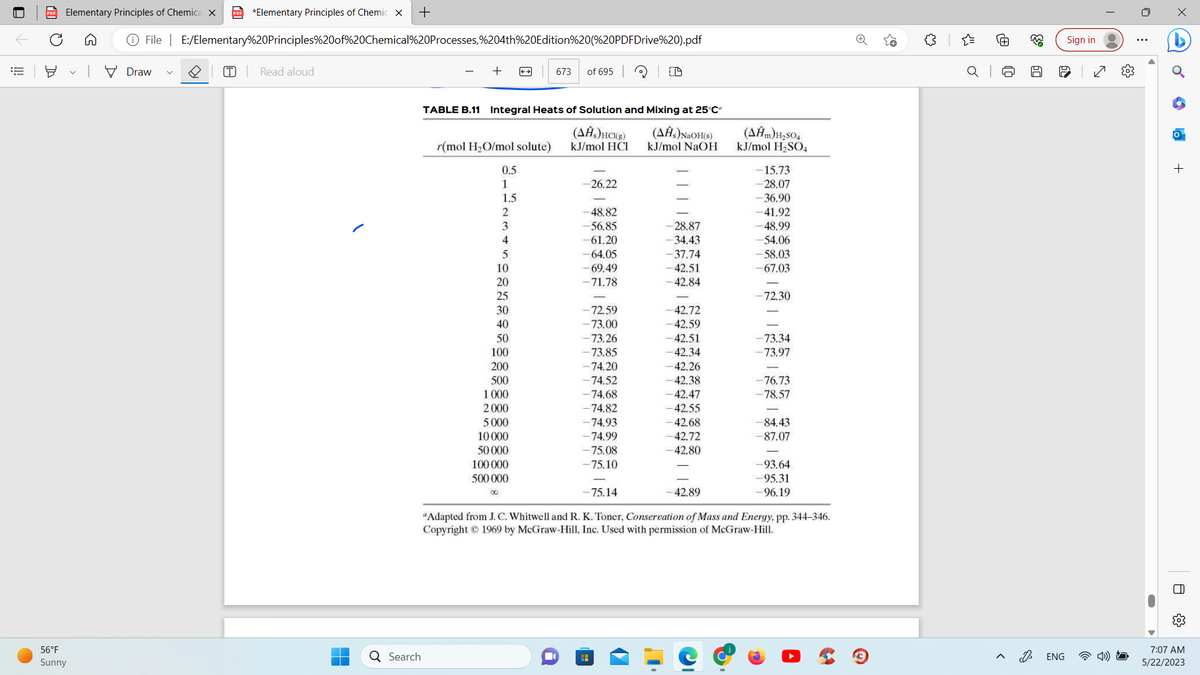
For enthalpy 2, the number -63.79 kJ/mol H2SO4 was used for the calculation. How was this number obtained? I do not see it on the table here. Was linear interpolation used to determine enthalpy 2?
For enthalpy 2, the number -63.79 kJ/mol H2SO4 was used for the calculation. How was this number obtained? I do not see it on the table here. Was linear interpolation used to determine enthalpy 2?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
For enthalpy 2, the number -63.79 kJ/mol H2SO4 was used for the calculation. How was this number obtained? I do not see it on the table here. Was linear interpolation used to determine enthalpy 2?
Transcribed Image Text:b Answered: Demonstrate the calcu X +
56°F
Sunny
✰ bartleby.com/questions-and-answers/demonstrate-the-calculations-for-enthalpies-1-2-and-3./b593a7c4-0e37-4275-af71-29efd148137e
Homework help starts here!
Q Search
=
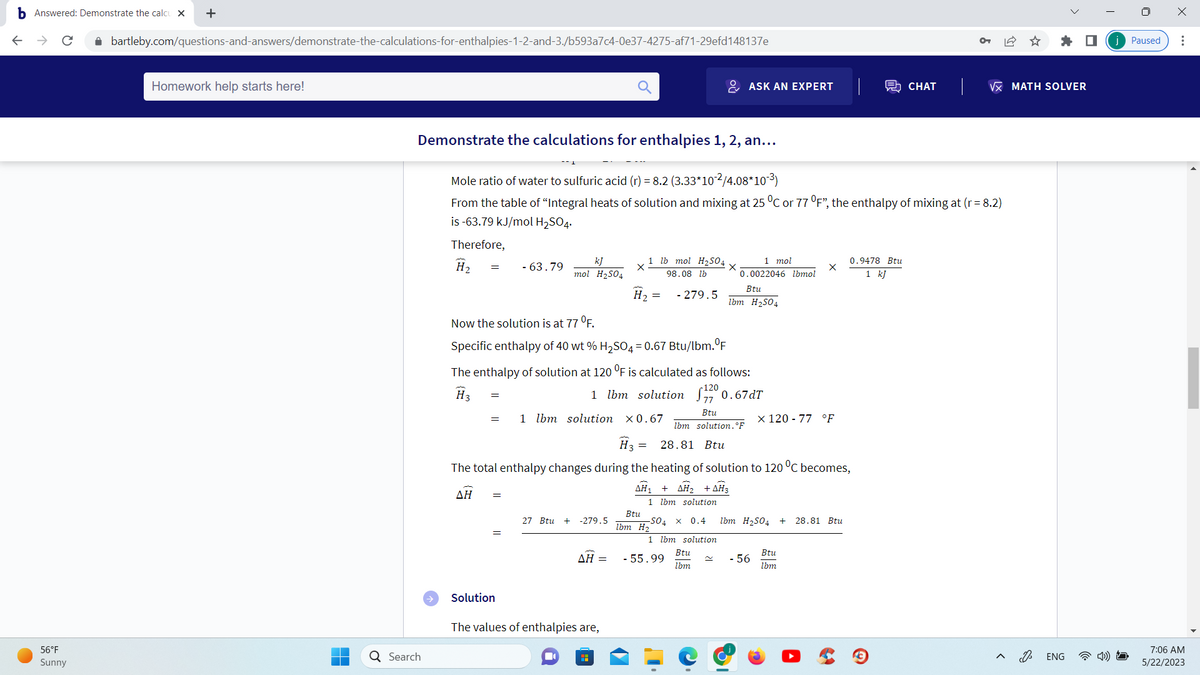
Demonstrate the calculations for enthalpies 1, 2, an...
Mole ratio of water to sulfuric acid (r) = 8.2 (3.33*10-²/4.08*10-³)
From the table of "Integral heats of solution and mixing at 25 °C or 77 °F", the enthalpy of mixing at (r= 8.2)
is -63.79 kJ/mol H₂SO4.
Therefore,
H₂
- 63.79
= 1 lbm solution
ΔΗ =
kJ
mol H₂SO4
Solution
27 Btu + -279.5
■
AH =
The values of enthalpies are,
X
Now the solution is at 77 °F.
Specific enthalpy of 40 wt % H₂SO4 = 0.67 Btu/lbm.°F
The enthalpy of solution at 120 °F is calculated as follows:
H3
1 lbm solution
1200.67dT
1 lb mol H₂SO4
98.08 lb
H
H₂ =
x 0.67
- 279.5
X
H3 = 28.81 Btu
The total enthalpy changes during the heating of solution to 120 °C becomes,
AH₁ + AH₂ + AH3
1 lbm solution
- 55.99
ASK AN EXPERT
1 mol
0.0022046 lbmol
Btu
lbm H₂SO4
77
Btu
lbm solution.°F
Btu
lbm
Btu
-SO4 x 0.4 lbm H₂SO4 + 28.81 Btu
lbm H₂
1 lbm solution
X
x 120 - 77 °F
- 56
Btu
1bm
0.9478 Btu
1 kJ
CHAT
√x MATH SOLVER
☐
ENG
j Paused
x
:
7:06 AM
5/22/2023
Transcribed Image Text:||!
PDF Elementary Principles of Chemica X PDF *Elementary Principles of Chemic X +
56°F
Sunny
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
T Read aloud
Q Search
r(mol H₂O/mol solute)
0.5
1
1.5
2
3
4
5
TABLE B.11 Integral Heats of Solution and Mixing at 25°C²
(AĤs) NaOH(s)
kJ/mol NaOH
10
20
25
30
40
50
100
200
500
1000
2000
5000
10 000
50 000
100 000
500 000
673 of 695
■
(AĤs) HCK(g)
kJ/mol HCI
-26.22
-48.82
-56.85
-61.20
-64.05
-69.49
-71.78
-72.59
-73.00
-73.26
-73.85
-74.20
-74.52
-74.68
-74.82
-74.93
-74.99
-75.08
-75.10
CD
-75.14
28.87
-34.43
-37.74
-42.51
-42.84
42.72
-42.59
-42.51
42.34
-42.26
-42.38
-42.47
-42.55
-42.68
-42.72
-42.80
-42.89
(AĤm) H₂SO4
kJ/mol H₂SO4
-15.73
-28.07
- 36.90
-41.92
- 48.99
- 54.06
-58.03
-67.03
-72.30
-73.34
-73.97
-76.73
-78.57
-84.43
-87.07
-93.64
-95.31
-96.19
"Adapted from J. C. Whitwell and R. K. Toner, Conservation of Mass and Energy, pp. 344-346.
Copyright © 1969 by McGraw-Hill, Inc. Used with permission of McGraw-Hill.
{"
J
63
60
D
ENG
Sign in
(0)
+
O
7:07 AM
5/22/2023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The