1) Conjugate Acid/Base Pairs - Draw the conjugate Acid-Base pair for each reaction, and indicate if the reaction will happen or not by circling YES or NO. + pKa = 5 Acid-Base Pair H Jan :0: pka = 19 CH3 CH3 CH3 protonate/deprotonate +H/-H + + +H / -H +H/-H +H / -H + +H/-H Conjugate Acid-Base Pair Will they React? YES NO YES NO YES NO YES NO
1) Conjugate Acid/Base Pairs - Draw the conjugate Acid-Base pair for each reaction, and indicate if the reaction will happen or not by circling YES or NO. + pKa = 5 Acid-Base Pair H Jan :0: pka = 19 CH3 CH3 CH3 protonate/deprotonate +H/-H + + +H / -H +H/-H +H / -H + +H/-H Conjugate Acid-Base Pair Will they React? YES NO YES NO YES NO YES NO
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 75AP
Related questions
Question
100%
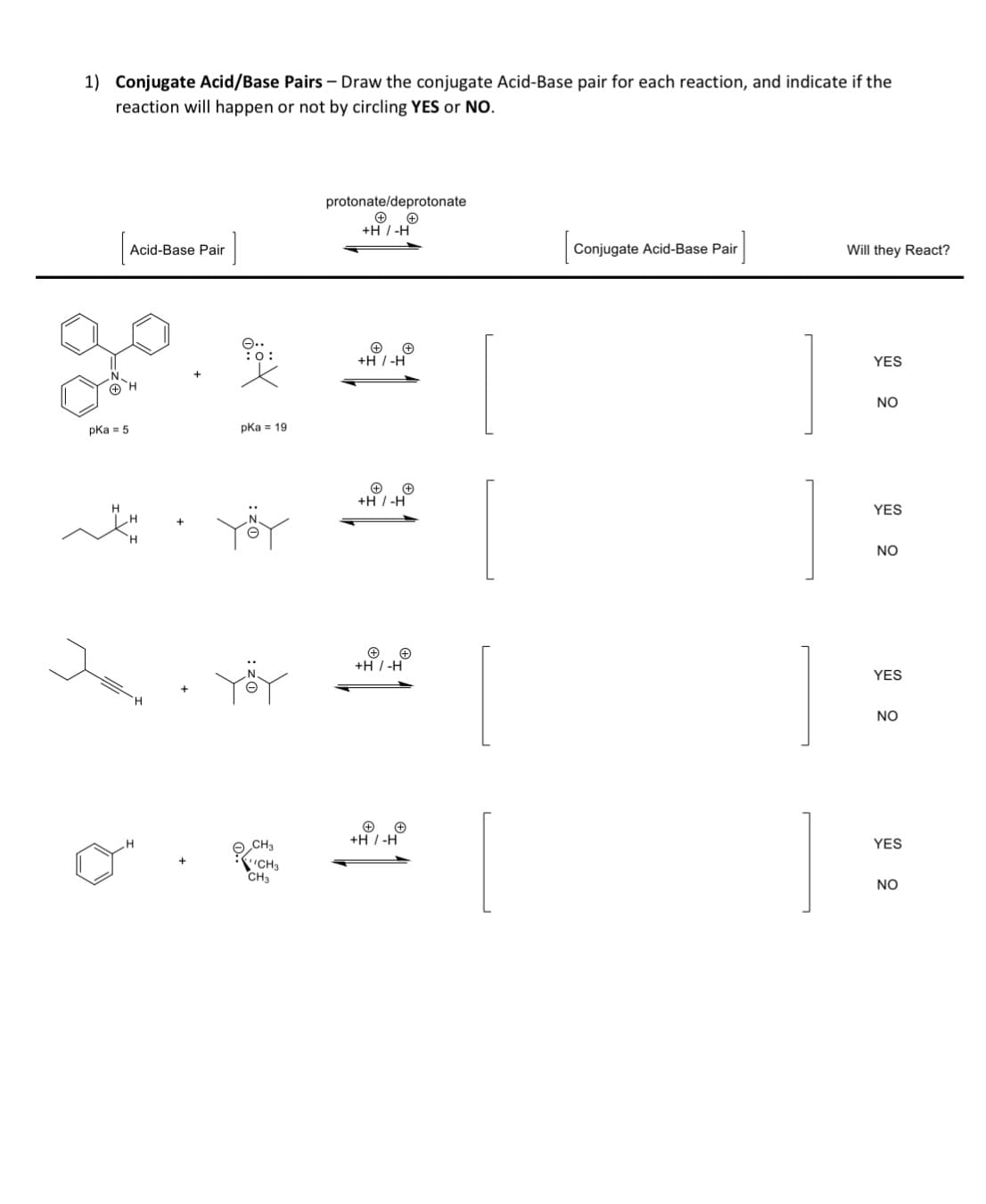
Transcribed Image Text:1) Conjugate Acid/Base Pairs - Draw the conjugate Acid-Base pair for each reaction, and indicate if the
reaction will happen or not by circling YES or NO.
pka = 5
Acid-Base Pair
pka 19
CH3
CH3
CH3
protonate/deprotonate
+
+H / -H
+H/-H
+
+H/-H
+H/-H
+
+H/-H
Conjugate Acid-Base Pair
Will they React?
YES
NO
YES
NO
YES
NO
YES
NO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
For problem 3, why does N keep its electrons in addition to taking the H+ from the other molecule? Didn't it give its electrons to the other molecule?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
