For the decomposition of Ag2O: 2Ag2O(s)-4Ag(s)+O2(g) (a) Obtain an expression for AG° as a function of temperature. Prepare a table of AG° values at 100 K intervals between 100 K and 500 K. (b) Calculate the temperature at which AG°= 0.
For the decomposition of Ag2O: 2Ag2O(s)-4Ag(s)+O2(g) (a) Obtain an expression for AG° as a function of temperature. Prepare a table of AG° values at 100 K intervals between 100 K and 500 K. (b) Calculate the temperature at which AG°= 0.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.104QE: What is the sign of the standard Gibbs free-energy change at low temperatures and at high...
Related questions
Question
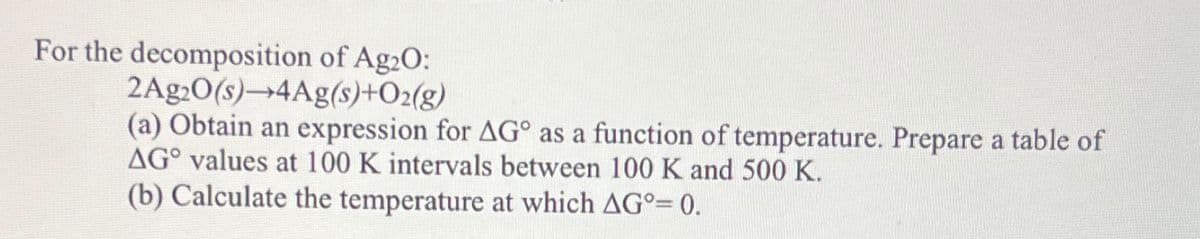
Transcribed Image Text:For the decomposition of Ag2O:
2Ag2O(s)-4Ag(s)+O2(g)
(a) Obtain an expression for AG° as a function of temperature. Prepare a table of
AG° values at 100 K intervals between 100 K and 500 K.
(b) Calculate the temperature at which AG°= 0.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 6 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning