6. Answer the following questions that relate to chemical bonding. (a) Two Lewis structures can be drawn for the OPF3 molecule, as shown below. :Ö: :F-P-F: :F-P-F: :F: :F: Structure 1 Structure 2 (i) (ii) How many sigma and how many pi bonds are in Structure 1? 4 and 1 σ Does Structure 1 demonstrate resonance? Justify your decision. No it does not demonstrate resonance because the double bond is w/ the there oxygen mic and disn't another oxygen mild to transfer the bond to. (iii) Which one of the two structures best represents a molecule of OPF3? Justify your answer in terms of formal charge.
6. Answer the following questions that relate to chemical bonding. (a) Two Lewis structures can be drawn for the OPF3 molecule, as shown below. :Ö: :F-P-F: :F-P-F: :F: :F: Structure 1 Structure 2 (i) (ii) How many sigma and how many pi bonds are in Structure 1? 4 and 1 σ Does Structure 1 demonstrate resonance? Justify your decision. No it does not demonstrate resonance because the double bond is w/ the there oxygen mic and disn't another oxygen mild to transfer the bond to. (iii) Which one of the two structures best represents a molecule of OPF3? Justify your answer in terms of formal charge.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter1: Bond Angles And Shape
Section: Chapter Questions
Problem 12CTQ: Consider the following flat drawing of methane (CH4) . a. What is HCH bond angle implied by this...
Related questions
Question
how would you answer part III of this question? This is a non-graded practice FRQ. 
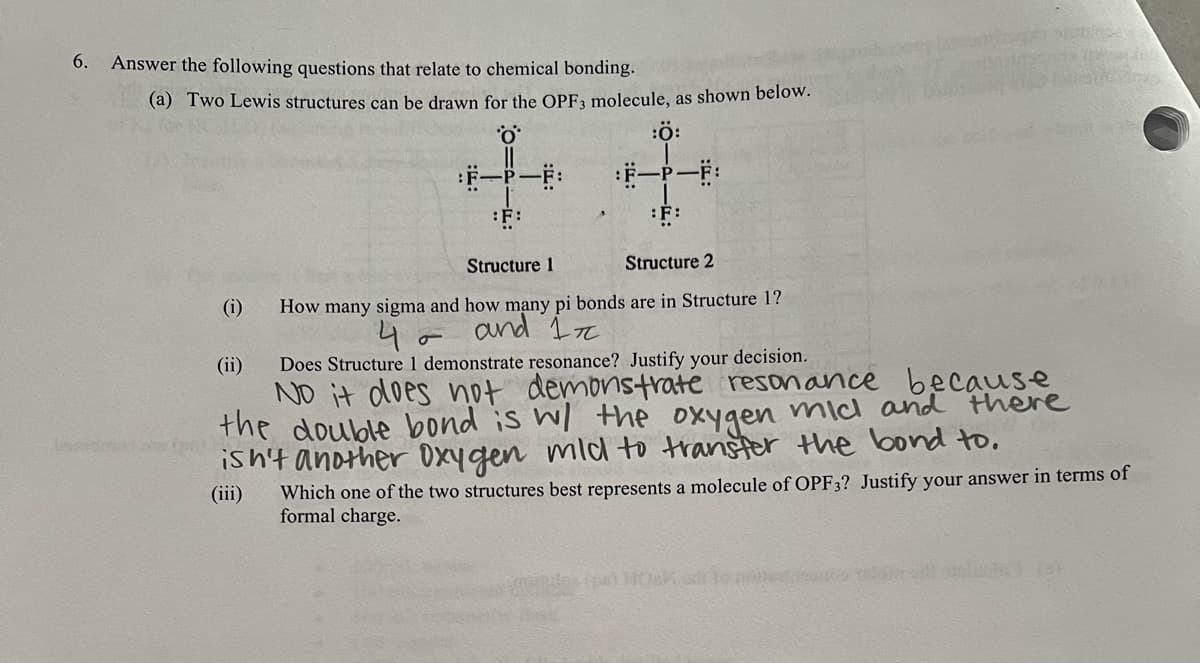
Transcribed Image Text:6.
Answer the following questions that relate to chemical bonding.
(a) Two Lewis structures can be drawn for the OPF3 molecule, as shown below.
:Ö:
:F-P-F:
:F-P-F:
:F:
:F:
Structure 1
Structure 2
(i)
(ii)
How many sigma and how many pi bonds are in Structure 1?
4 and 1
σ
Does Structure 1 demonstrate resonance? Justify your decision.
No it does not
demonstrate resonance because
the double bond is w/ the
there
oxygen mic and
disn't another oxygen mild to transfer the bond to.
(iii) Which one of the two structures best represents a molecule of OPF3? Justify your answer in terms of
formal charge.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning