Please refer to the images as much as you need to.
For the following hydrocarbons, pick out 5 wavenumbers for each hydrocarbon, and give it its probable assignment for its structure. You may find this link useful:
https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table
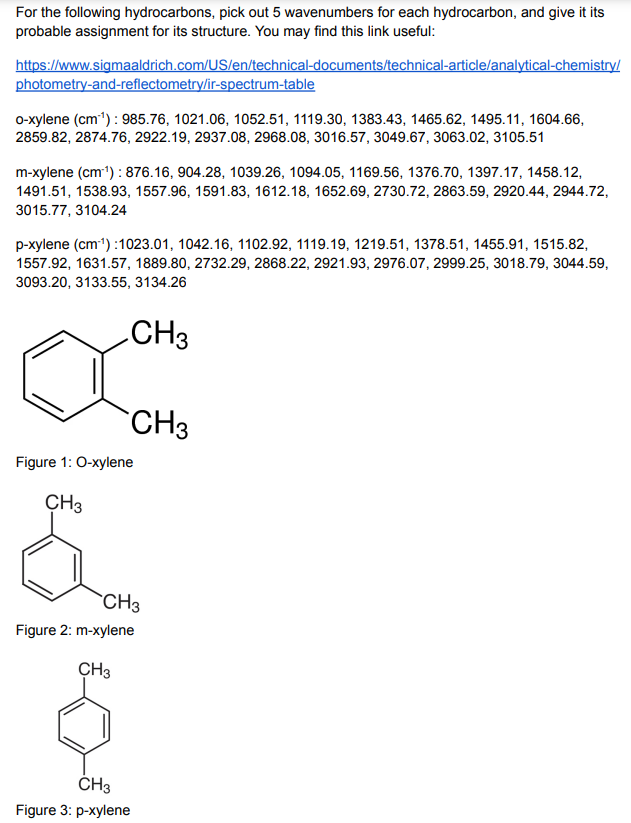
o-xylene (cm-1) : 985.76, 1021.06, 1052.51, 1119.30, 1383.43, 1465.62, 1495.11, 1604.66, 2859.82, 2874.76, 2922.19, 2937.08, 2968.08, 3016.57, 3049.67, 3063.02, 3105.51
m-xylene (cm-1) : 876.16, 904.28, 1039.26, 1094.05, 1169.56, 1376.70, 1397.17, 1458.12, 1491.51, 1538.93, 1557.96, 1591.83, 1612.18, 1652.69, 2730.72, 2863.59, 2920.44, 2944.72, 3015.77, 3104.24
p-xylene (cm-1) :1023.01, 1042.16, 1102.92, 1119.19, 1219.51, 1378.51, 1455.91, 1515.82, 1557.92, 1631.57, 1889.80, 2732.29, 2868.22, 2921.93, 2976.07, 2999.25, 3018.79, 3044.59, 3093.20, 3133.55, 3134.26
Step by step
Solved in 4 steps






