For the following reaction, 5.46 grams of perchloric acid (HCIO4) are mixed with excess tetraphosphorus decaoxide. The reaction yields 1.26 grams of phosphoric acid. 12HCIO4 (aq) + P4010 (s) → 4H3PO4 (aq) + 6Cl207 1). (1) What is the theoretical yield of phosphoric acid? grams (2) What is the percent yield for this reaction?
For the following reaction, 5.46 grams of perchloric acid (HCIO4) are mixed with excess tetraphosphorus decaoxide. The reaction yields 1.26 grams of phosphoric acid. 12HCIO4 (aq) + P4010 (s) → 4H3PO4 (aq) + 6Cl207 1). (1) What is the theoretical yield of phosphoric acid? grams (2) What is the percent yield for this reaction?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.44PAE: 4.44 Industrial production of hydrogen gas uses the reaction shown below. If 1.00 metric ton of...
Related questions
Question
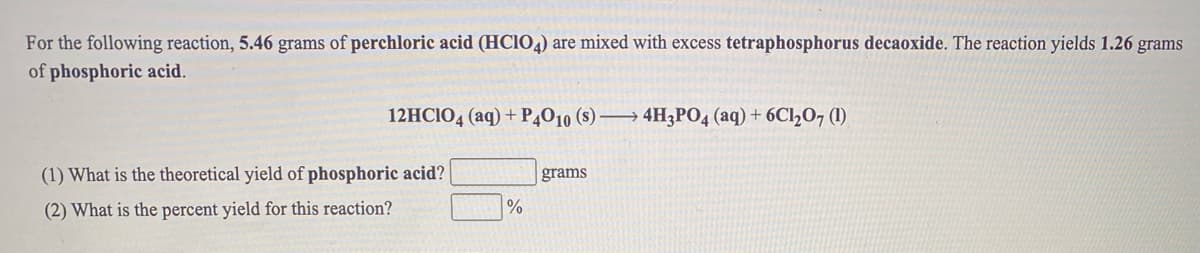
Transcribed Image Text:For the following reaction, 5.46 grams of perchloric acid (HCIO4) are mixed with excess tetraphosphorus decaoxide. The reaction yields 1.26 grams
of phosphoric acid.
12HCIO4 (aq) + P4010 (s) → 4H3PO4 (aq) + 6C1½07 1).
(1) What is the theoretical yield of phosphoric acid?
grams
(2) What is the percent yield for this reaction?
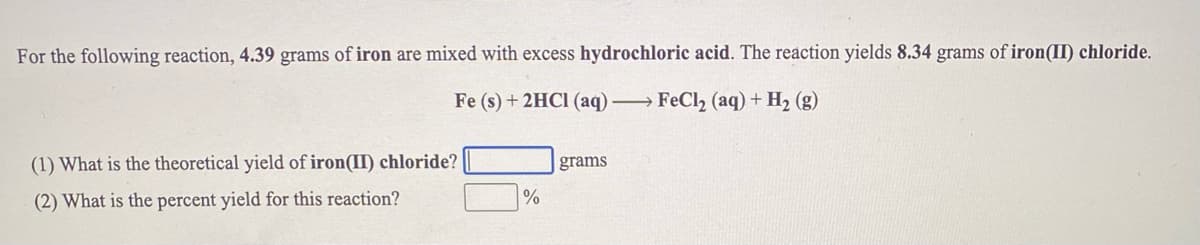
Transcribed Image Text:For the following reaction, 4.39 grams of iron are mixed with excess hydrochloric acid. The reaction yields 8.34 grams of iron(II) chloride.
Fe (s) + 2HCI (aq) →FECI2 (aq) + H2 (g)
(1) What is the theoretical yield of iron(II) chloride?
grams
(2) What is the percent yield for this reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning