For the overall chemical reaction shown below, which one of the following statements can be rightly assumed? 2H2Sg) - 02l) - 25() + 2H20) Muhiple Choice The reaction is thid- order overall. The rate law cannot be denermned rom the informanon gven The rete law is, rete 28 (02) The reaction is second-order overal The rete law , rate2 (02
For the overall chemical reaction shown below, which one of the following statements can be rightly assumed? 2H2Sg) - 02l) - 25() + 2H20) Muhiple Choice The reaction is thid- order overall. The rate law cannot be denermned rom the informanon gven The rete law is, rete 28 (02) The reaction is second-order overal The rete law , rate2 (02
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 98CWP: A certain reaction has the form aAProducts At a particular temperature, concentration versus time...
Related questions
Question
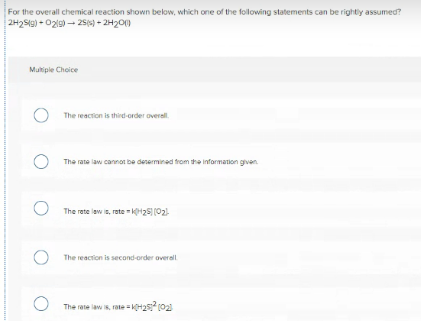
Transcribed Image Text:For the overall chemical reaction shown below, which one of the following statements can be rightly assumed?
2H2S9) - 02l9) - 25s) + 2H20)
Multiple Choice
The reaction is third-order overall.
The rate law cannot be desermined trom the information gven.
The rete law is, rete kH25 (02)
The reaction is second-order overall
The rate law is, rate H2 (02
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning