For the questions of the Experiment #13, complete and balance the following chemical equations with proper coefficient(s). For example, if a coefficient is number 1, put just 1 as a number and do not put anything else before and after. type your answer... H3PO4(aq) + type your answer... Ba(OH)2(aq) →> type your answer... type your answer... + type your answer... type your answer...
For the questions of the Experiment #13, complete and balance the following chemical equations with proper coefficient(s). For example, if a coefficient is number 1, put just 1 as a number and do not put anything else before and after. type your answer... H3PO4(aq) + type your answer... Ba(OH)2(aq) →> type your answer... type your answer... + type your answer... type your answer...
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter6: Chemical Reactions: An Introduction
Section6.3: Balancing Chemical Equations
Problem 1CT
Related questions
Question
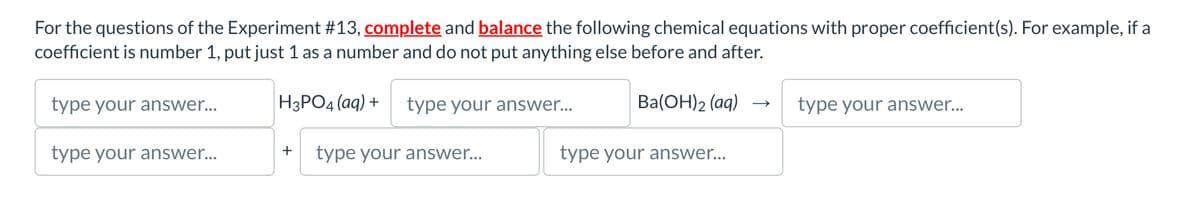
Transcribed Image Text:For the questions of the Experiment #13, complete and balance the following chemical equations with proper coefficient(s). For example, if a
coefficient is number 1, put just 1 as a number and do not put anything else before and after.
type your answer...
H3PO4(aq) +
type your answer...
Ba(OH)2(aq) →>
type your answer...
type your answer...
+
type your answer...
type your answer...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div