For the reaction 3 KCIO3 KCI 2 assign oxidation numbers to each element on each side of the equation. K in KCIO3 K in KCl: Cl in KCIO3 Cl in KCl: O in O2 O in KCIO3 Which element is reduced? Which element is oxidized? Cl Ок Ок OC L
For the reaction 3 KCIO3 KCI 2 assign oxidation numbers to each element on each side of the equation. K in KCIO3 K in KCl: Cl in KCIO3 Cl in KCl: O in O2 O in KCIO3 Which element is reduced? Which element is oxidized? Cl Ок Ок OC L
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter18: Oxidation–reduction Reactions And Electrochemistry
Section: Chapter Questions
Problem 6ALQ
Related questions
Question
100%
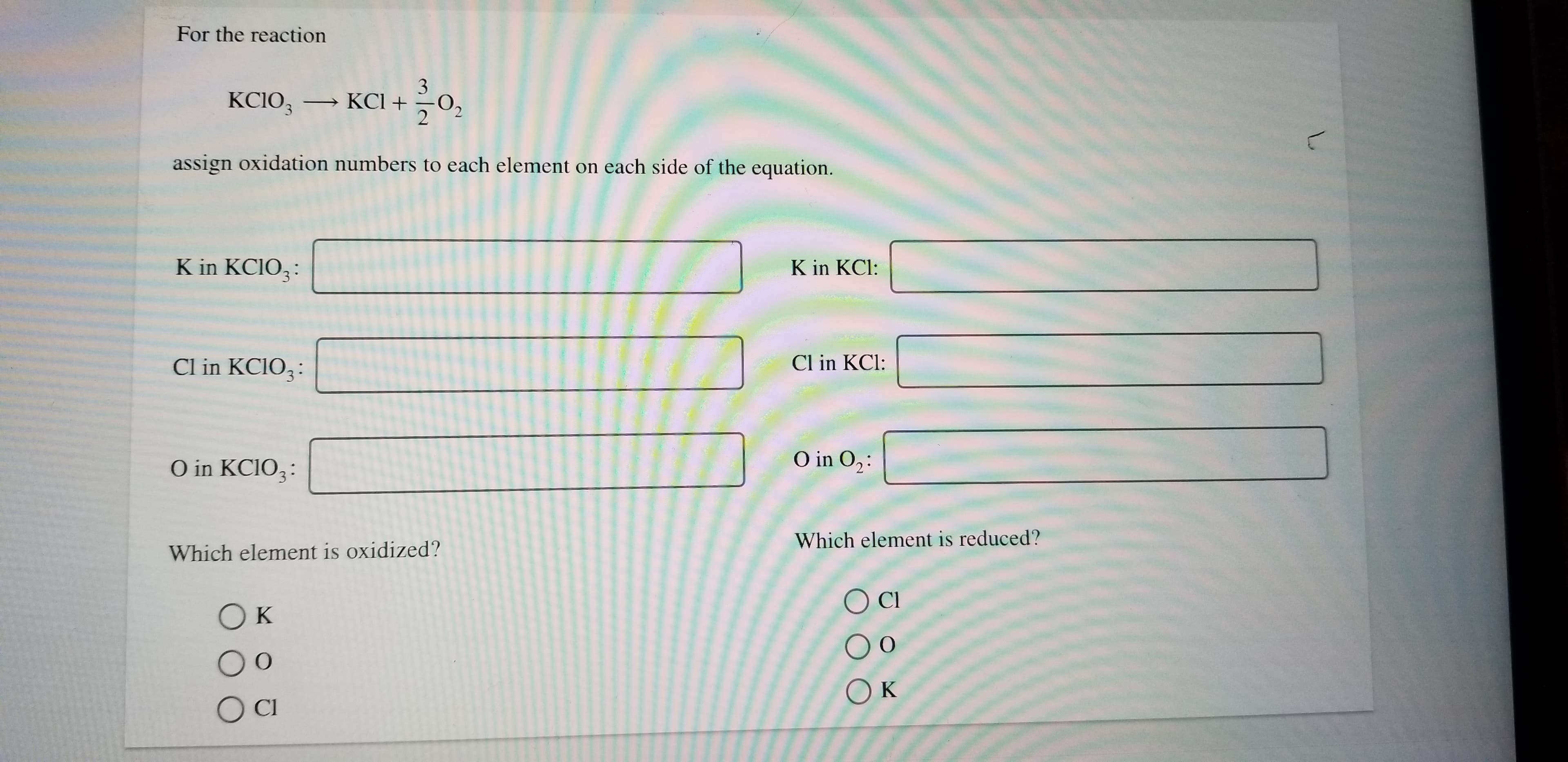
Transcribed Image Text:For the reaction
3
KCIO3 KCI
2
assign oxidation numbers to each element on each side of the equation.
K in KCIO3
K in KCl:
Cl in KCIO3
Cl in KCl:
O in O2
O in KCIO3
Which element is reduced?
Which element is oxidized?
Cl
Ок
Ок
OC
L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
