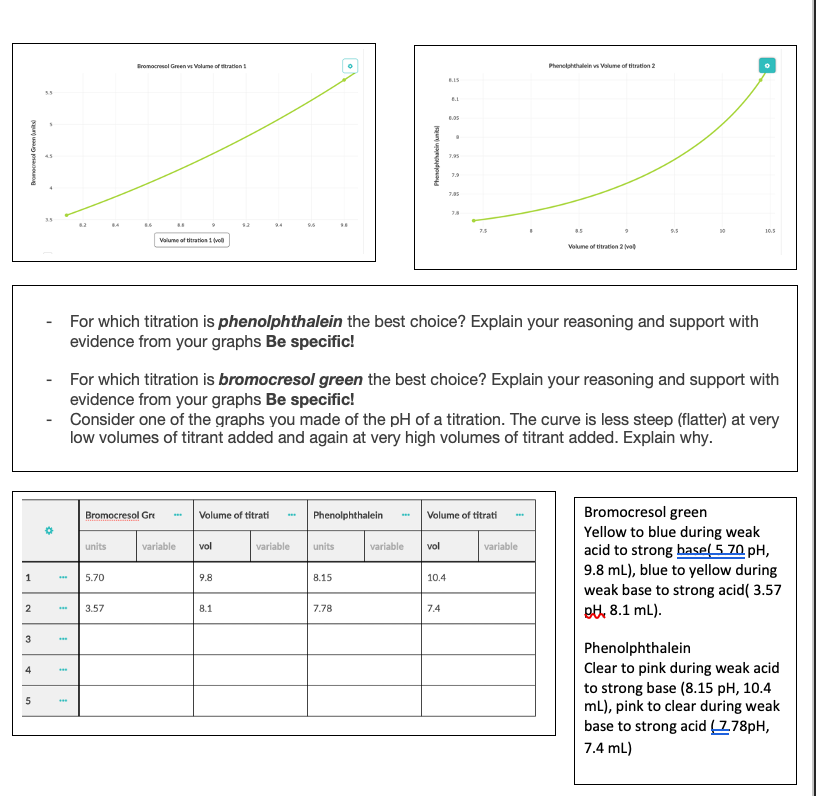
For which titration is phenolphthalein the best choice? Explain your reasoning and support with evidence from your graphs Be specific! For which titration is bromocresol green the best choice? Explain your reasoning and support with evidence from your graphs Be specific!
For which titration is phenolphthalein the best choice? Explain your reasoning and support with evidence from your graphs Be specific! For which titration is bromocresol green the best choice? Explain your reasoning and support with evidence from your graphs Be specific!
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.25QAP
Related questions
Question
100%
Transcribed Image Text:Bromocresel Green v Volame of ratien
Phenclphthalein vs Volume of titration 2
EIS
7.95
9.5
10.5
Valume af titration 1 vell
Volume of titratien 2 vo
For which titration is phenolphthalein the best choice? Explain your reasoning and support with
evidence from your graphs Be specific!
- For which titration is bromocresol green the best choice? Explain your reasoning and support with
evidence from your graphs Be specific!
- Consider one of the graphs you made of the pH of a titration. The curve is less steep (flatter) at very
low volumes of titrant added and again at very high volumes of titrant added. Explain why.
Bromocresol green
Yellow to blue during weak
acid to strong base(5 70 pH,
9.8 mL), blue to yellow during
weak base to strong acid( 3.57
Bromocresol Gre
Volume of titrati
Phenolphthalein
Volume of titrati
units
variable
vol
variable
units
variable
vol
variable
5.70
9.8
8.15
10.4
pt, 8.1 mL).
3.57
8.1
7.78
7.4
3
Phenolphthalein
Clear to pink during weak acid
to strong base (8.15 pH, 10.4
ml), pink to clear during weak
base to strong acid (178pH,
7.4 mL)
4
5
igun u udjouou4
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning