Formation of soluble silver-ammonium complex, in an AgCl solution, occurs in a 3-step process: AgCI(s) Ag* (aq) + CI(aq) Kup Ag*(aq) + NH,(aq) = AGNH, (aq) AGNH, (aq) + NH,(aq) Ag(NH,), (aq) K2 K, where K, is the solubility product for AGCI and K, & K2 are the equilibrium constants for the complex formation. The over-all formation constant (K,) for the Ag(NH,), complex is equal to K, x K2.
Formation of soluble silver-ammonium complex, in an AgCl solution, occurs in a 3-step process: AgCI(s) Ag* (aq) + CI(aq) Kup Ag*(aq) + NH,(aq) = AGNH, (aq) AGNH, (aq) + NH,(aq) Ag(NH,), (aq) K2 K, where K, is the solubility product for AGCI and K, & K2 are the equilibrium constants for the complex formation. The over-all formation constant (K,) for the Ag(NH,), complex is equal to K, x K2.
Chapter6: The Systematic Approach To Equilibria: Solving Many Equations
Section: Chapter Questions
Problem 10P
Related questions
Question
Based on the chemical equation provided, which of the following is equivalent to K2?
a. [Ag+] [Cl-]
b. [AgNH3] / {[Ag+][NH3]}
c. [Ag(NH3)2+] / {[Ag(NH3)+][NH3]}
d. [Ag(NH3)2+] / {[Ag+][NH3+]2}
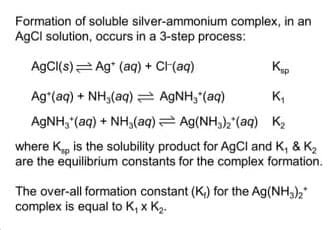
Transcribed Image Text:Formation of soluble silver-ammonium complex, in an
AgCl solution, occurs in a 3-step process:
AgCI(s)= Ag* (aq) + CH(aq)
Ag*(aq) + NH,(aq) AGNH, (aq)
AGNH, (aq) + NH,(aq) = Ag(NH3)2'(aq) K2
K,
where K, is the solubility product for AgCl and K, & K2
are the equilibrium constants for the complex formation.
The over-all formation constant (K,) for the Ag(NH,),"
complex is equal to K, x K2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

