Four research teams measured the density of a new alloy, and what each team wrote in its team notebook is shown in the table below. Suppose a later and more reliable measurement gives 10.7 g cm³ for the density of the same alloy. Decide which of the earlier measurements was the most accurate, and which was the most precise. team A B C D what was written in the notebook *8.7g/cm³ "9.7g/cm² ± 0.2g cm² 10.9g/cm² ± 9.0% "between 11.2g/cm² and 12.2g cm²³- most accurate most precise measurement measurement O O O 0 000 X 5
Four research teams measured the density of a new alloy, and what each team wrote in its team notebook is shown in the table below. Suppose a later and more reliable measurement gives 10.7 g cm³ for the density of the same alloy. Decide which of the earlier measurements was the most accurate, and which was the most precise. team A B C D what was written in the notebook *8.7g/cm³ "9.7g/cm² ± 0.2g cm² 10.9g/cm² ± 9.0% "between 11.2g/cm² and 12.2g cm²³- most accurate most precise measurement measurement O O O 0 000 X 5
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 102GQ: Cloth can be waterproofed by coating it with a silicone layer. This is done by exposing the cloth to...
Related questions
Question
Please answer all parts or leave for someone else to answer
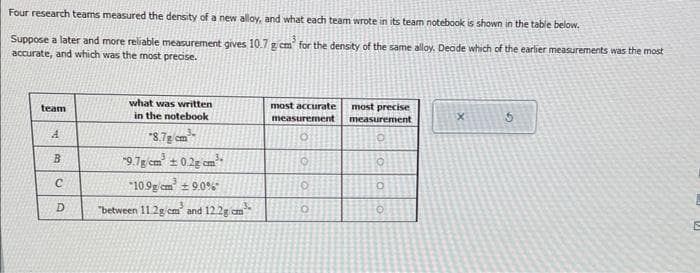
Transcribed Image Text:Four research teams measured the density of a new alloy, and what each team wrote in its team notebook is shown in the table below.
Suppose a later and more reliable measurement gives 10.7 g cm³ for the density of the same alloy. Decide which of the earlier measurements was the most
accurate, and which was the most precise.
team
A
B
C
D
what was written
in the notebook
*8.7g/cm³
9.7g/cm² ± 0.2g cm²
10.9 cm² ±9.0%
"between 11.2g cm² and 12.2g cm³-
most accurate most precise
measurement
measurement
O
O
O
0
000
O
X
5
E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole