From kangaroo-tail collagen you have identified a series of substituted prolines. From the titration curves you deterine the pKa's of the ionizable groups as follows: Carboxyproline (pKa's 2.0, 3.8, 10.5), Aminoproline (pKa's 1.9, 9.8, 10.0 Sulfhydrylproline (pKa's 1.9, 6.0, 9.0), Hydroxyproline (pKa's 2.0, 9.0), Methylproline (pKa's 1.8, 8.8). If these fiv Proline derivatives are dissolved in a buffer system (pH = 8.5) and an electronic field is applied, which of them will mic ate to the cathode? %3D Answers A -E A Hydroxyproline B Aminoproline C Sulfhydrylproline D Carboxyproline E Methylproline
From kangaroo-tail collagen you have identified a series of substituted prolines. From the titration curves you deterine the pKa's of the ionizable groups as follows: Carboxyproline (pKa's 2.0, 3.8, 10.5), Aminoproline (pKa's 1.9, 9.8, 10.0 Sulfhydrylproline (pKa's 1.9, 6.0, 9.0), Hydroxyproline (pKa's 2.0, 9.0), Methylproline (pKa's 1.8, 8.8). If these fiv Proline derivatives are dissolved in a buffer system (pH = 8.5) and an electronic field is applied, which of them will mic ate to the cathode? %3D Answers A -E A Hydroxyproline B Aminoproline C Sulfhydrylproline D Carboxyproline E Methylproline
Chapter26: Biomolecules: Amino Acids, Peptides, And Proteins
Section26.SE: Something Extra
Problem 28MP
Related questions
Question
5
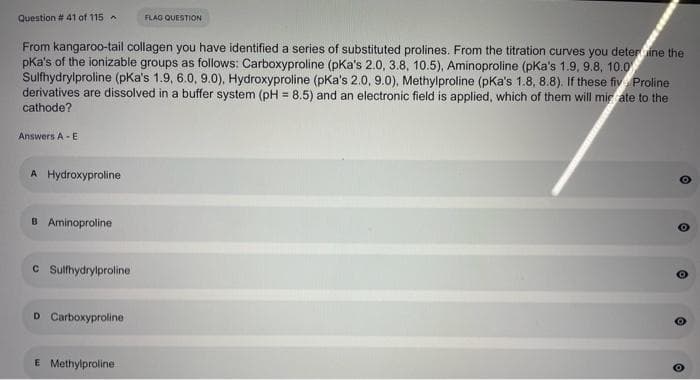
Transcribed Image Text:Question # 41 aof 115
FLAG QUESTION
From kangaroo-tail collagen you have identified a series of substituted prolines. From the titration curves you deterine the
pKa's of the ionizable groups as follows: Carboxyproline (pKa's 2.0, 3.8, 10.5), Aminoproline (pKa's 1.9, 9.8, 10.0
Sulfhydrylproline (pKa's 1.9, 6.0, 9.0), Hydroxyproline (pKa's 2.0, 9.0), Methylproline (pKa's 1.8, 8.8). If these fiv Proline
derivatives are dissolved in a buffer system (pH = 8.5) and an electronic field is applied, which of them will mic ate to the
%3D
cathode?
Answers A -E
A Hydroxyproline
B Aminoproline
C Sulfhydrylproline
D Carboxyproline
E Methylproline
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning