From the given table calculate- a) ratio of moles of magnesium to moles of oxygen b) experimental % of magnesium in product c) theoretical mass % based on actual formula of magnesium oxide
From the given table calculate- a) ratio of moles of magnesium to moles of oxygen b) experimental % of magnesium in product c) theoretical mass % based on actual formula of magnesium oxide
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter16: Spontaneity Of Reaction
Section: Chapter Questions
Problem 48QAP
Related questions
Question
From the given table calculate-
a) ratio of moles of magnesium to moles of oxygen
b) experimental % of magnesium in product
c) theoretical mass % based on actual formula of magnesium oxide
d) percent error in % Mg
e) State two sources of error that might account for a high percentage error in % Mg.
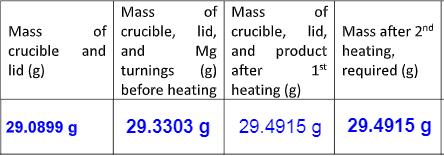
Transcribed Image Text:Mass
of Mass
of
of crucible, lid, crucible, lid, Mass after 2nd
Mg and product heating,
1st required (g)
Mass
crucible and and
lid (g)
turnings (g) after
before heating heating (g)
29.0899 g
29.3303 g 29.4915 g 29.4915 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole