From the list of acid-base indicators below, select the one BEST indicator for a titration with an equivalence point at pH 4.0 (BE CAREFUL-enter indicator name EXACTLY as it appears in table): pKa 0.80 Indicator Acid color Base color Yellow Methyl violet Blue Yellow Cresol red Malachite green Thymol blue Phloxine Orange IV Benzopurpurin Methyl yellow Methyl orange Bromophenol blue Congo red Dinitrophenol Alpha-Naphthyl red Bromocresol green Methyl red Ethyl red Azolitmin (litmus) Bromocresol purple Bromothymol blue Nitrophenol Phenol red 1.0 Red Blue-green Yellow 1.3 Yellow 1.7 Red 1.8 Colorless Pink 2.1 Red Yellow 2.8 Violet Red 3.3 Red Yellow 3.7 Red Yellow 4.0 Yellow Blue-violet 4.1 Blue Red 4.1 Colorless Yellow 4.3 Red Yellow 4.7 Yellow Blue 5.1 Red Yellow red Blue 5.4 Colorless 5.8 Red 6.3 Yellow Violet 7.0 Yellow Blue 7.2 Yellow Colorless Yellow Yellow Colorless Yellow 7.9 Red Thymol blue Phenolphthalein Alizarin yellow R 8.9 blue 9.4 Red 11.0 red
From the list of acid-base indicators below, select the one BEST indicator for a titration with an equivalence point at pH 4.0 (BE CAREFUL-enter indicator name EXACTLY as it appears in table): pKa 0.80 Indicator Acid color Base color Yellow Methyl violet Blue Yellow Cresol red Malachite green Thymol blue Phloxine Orange IV Benzopurpurin Methyl yellow Methyl orange Bromophenol blue Congo red Dinitrophenol Alpha-Naphthyl red Bromocresol green Methyl red Ethyl red Azolitmin (litmus) Bromocresol purple Bromothymol blue Nitrophenol Phenol red 1.0 Red Blue-green Yellow 1.3 Yellow 1.7 Red 1.8 Colorless Pink 2.1 Red Yellow 2.8 Violet Red 3.3 Red Yellow 3.7 Red Yellow 4.0 Yellow Blue-violet 4.1 Blue Red 4.1 Colorless Yellow 4.3 Red Yellow 4.7 Yellow Blue 5.1 Red Yellow red Blue 5.4 Colorless 5.8 Red 6.3 Yellow Violet 7.0 Yellow Blue 7.2 Yellow Colorless Yellow Yellow Colorless Yellow 7.9 Red Thymol blue Phenolphthalein Alizarin yellow R 8.9 blue 9.4 Red 11.0 red
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter25: Ph Measurements-buffers And Their Properties
Section: Chapter Questions
Problem 1ASA
Related questions
Question
7
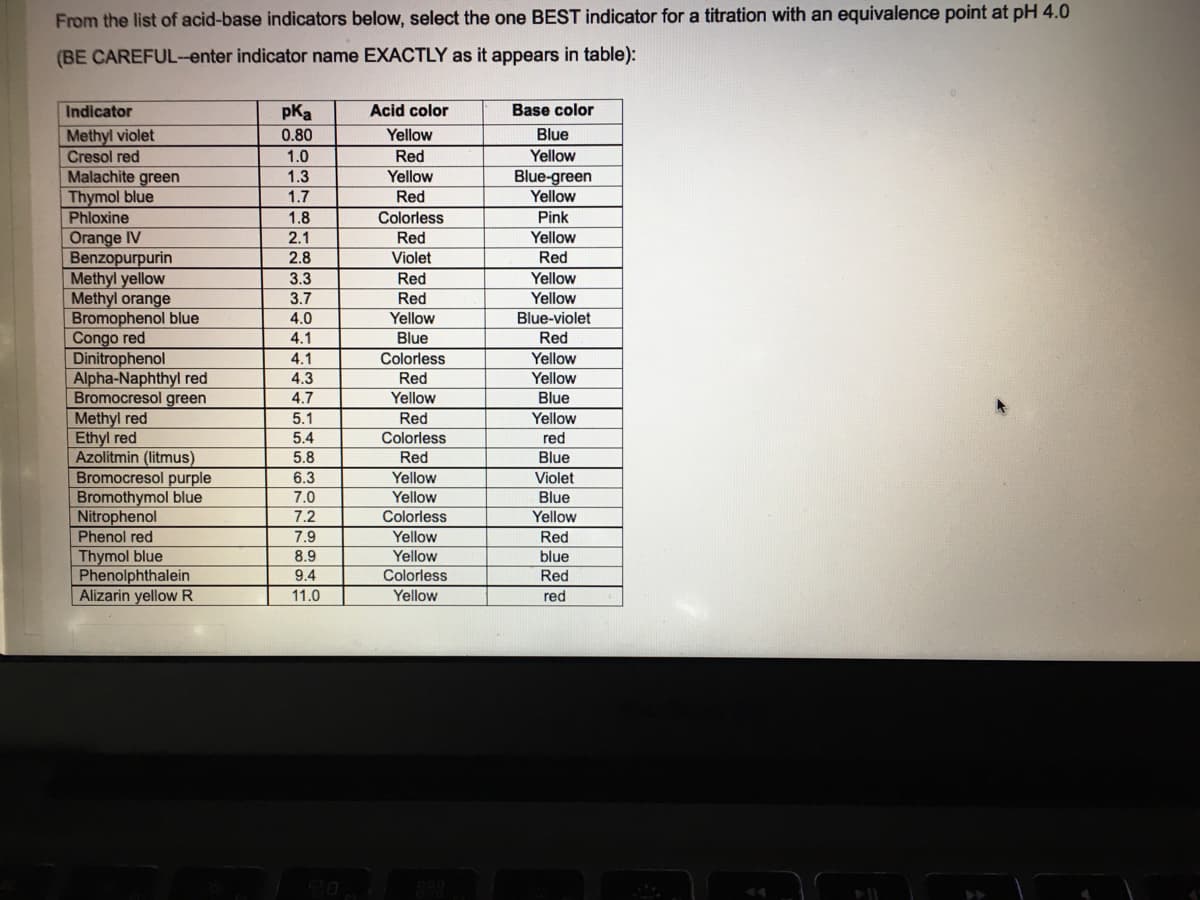
Transcribed Image Text:From the list of acid-base indicators below, select the one BEST indicator for a titration with an equivalence point at pH 4.0
(BE CAREFUL-enter indicator name EXACTLY as it appears in table):
Indicator
pKa
Acid color
Base color
Methyl violet
Cresol red
Malachite green
Thymol blue
Phloxine
Orange IV
Benzopurpurin
Methyl yellow
Methyl orange
Bromophenol blue
Congo red
Dinitrophenol
Alpha-Naphthyl red
Bromocresol green
Methyl red
Ethyl red
Azolitmin (litmus)
Yellow
Blue
Yellow
Blue-green
Yellow
0.80
1.0
Red
1.3
Yellow
1.7
Red
1.8
Colorless
Pink
2.1
Red
Yellow
Red
Yellow
Yellow
2.8
Violet
3.3
Red
3.7
Red
Yellow
4.0
Blue-violet
4.1
Blue
Red
4.1
Colorless
Yellow
4.3
Red
Yellow
4.7
Yellow
Blue
5.1
Red
Yellow
Colorless
Red
Yellow
5.4
red
5.8
Blue
Bromocresol purple
Bromothymol blue
Nitrophenol
Phenol red
6.3
Violet
7.0
Yellow
Blue
7.2
Colorless
Yellow
7.9
Yellow
Red
Thymol blue
Phenolphthalein
Alizarin yellow R
blue
8.9
9.4
Yellow
Colorless
Yellow
Red
11.0
red
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
