Chapter20: The Representative Elements
Section: Chapter Questions
Problem 70E
Related questions
Question
From these observations Are alkali metals or alkali earth matal more reactive?
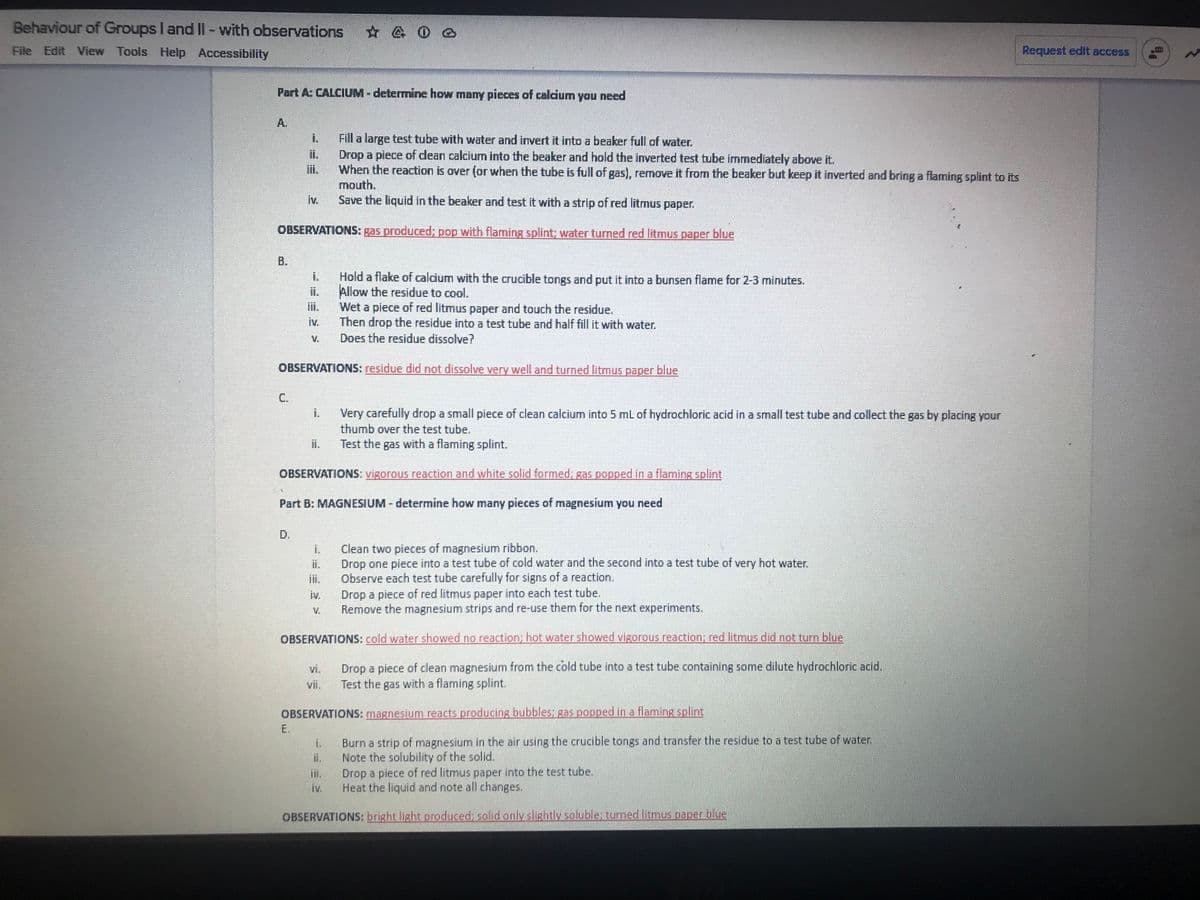
Transcribed Image Text:Behaviour of Groups I and II-with observations
File Edit View Tools Help Accessibility
Request edit access
Part A: CALCIUM - determine how many pieces of calcium you need
A.
Fill a large test tube with water and invert it into a beaker full of water.
Drop a piece of dean calcium into the beaker and hold the iverted test tube immediately above it.
When the reaction is over (or when the tube is full of gas), remove it from the beaker but keep it inverted and bring a flaming splint to its
mouth.
Save the liquid in the beaker and test it with a strip of red litmus paper.
i.
ii.
iii.
iv.
OBSERVATIONS: gas produced; pop with flaming splint; water turned red litmus paper blue
Hold a flake of calcium with the crucible tongs and put it into a bunsen flame for 2-3 minutes.
ii.
i.
Allow the residue to cool.
Wet a piece of red litmus paper and touch the residue.
iv.
ii.
Then drop the residue into a test tube and half fill it with water.
V.
Does the residue dissolve?
OBSERVATIONS: residue did not dissolve very well and turned litmus paper blue
C.
Very carefully drop a small piece of clean calcium into 5 mL of hydrochloric acid in a small test tube and collect the gas by placing your
thumb over the test tube.
i.
ii.
Test the gas with a flaming splint.
OBSERVATIONS: vigorous reaction and white solid formed; gas popped in a flaming splint
Part B: MAGNESIUM - determine how many pieces of magnesium you need
D.
i.
Clean two pieces of magnesium ribbon.
i.
Drop one piece into a test tube of cold water and the second into a test tube of very hot water.
Observe each test tube carefully for signs of a reaction.
Drop a piece of red litmus paper into each test tube.
Remove the magnesium strips and re-use them for the next experiments.
iv.
V.
OBSERVATIONS: cold water showed no reaction; hot water showed vigorous reaction: red litmus did not turn blue
Drop a piece of clean magnesium from the cold tube into a test tube containing some dilute hydrochloric acid.
Test the gas with a flaming splint.
vi.
vii.
OBSERVATIONS: magnesium reacts producing bubbles: gas popped in a flaming splint
E.
Burn a strip of magnesium in the air using the crucible tongs and transfer the residue to a test tube of water.
ii.
Note the solubility of the solid.
Drop a piece of red litmus paper into the test tube.
ii.
Heat the liquid and note all changes.
iv.
OBSERVATIONS: bright light produced; solidonly slightly soluble: turned litmus paper blue
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning