From this diagram, we know that the reaction (select all that apply): A) Is endothermic. B) Is exothermic. C) Will make products that resemble the transition state more closely than the reactants. D) Will make products that resemble the transition state less closely than the reactants. E) Will make products that resemble the transition state about the same as the reactants. F) The products will be more thermodynamically stable than the products. G) The reactants will be less thermodynamically stable than the products. H) None of the above
From this diagram, we know that the reaction (select all that apply): A) Is endothermic. B) Is exothermic. C) Will make products that resemble the transition state more closely than the reactants. D) Will make products that resemble the transition state less closely than the reactants. E) Will make products that resemble the transition state about the same as the reactants. F) The products will be more thermodynamically stable than the products. G) The reactants will be less thermodynamically stable than the products. H) None of the above
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.32E: A reaction is run at 10C and takes 3.7hours to go to completion. How long would it take to complete...
Related questions
Question
s
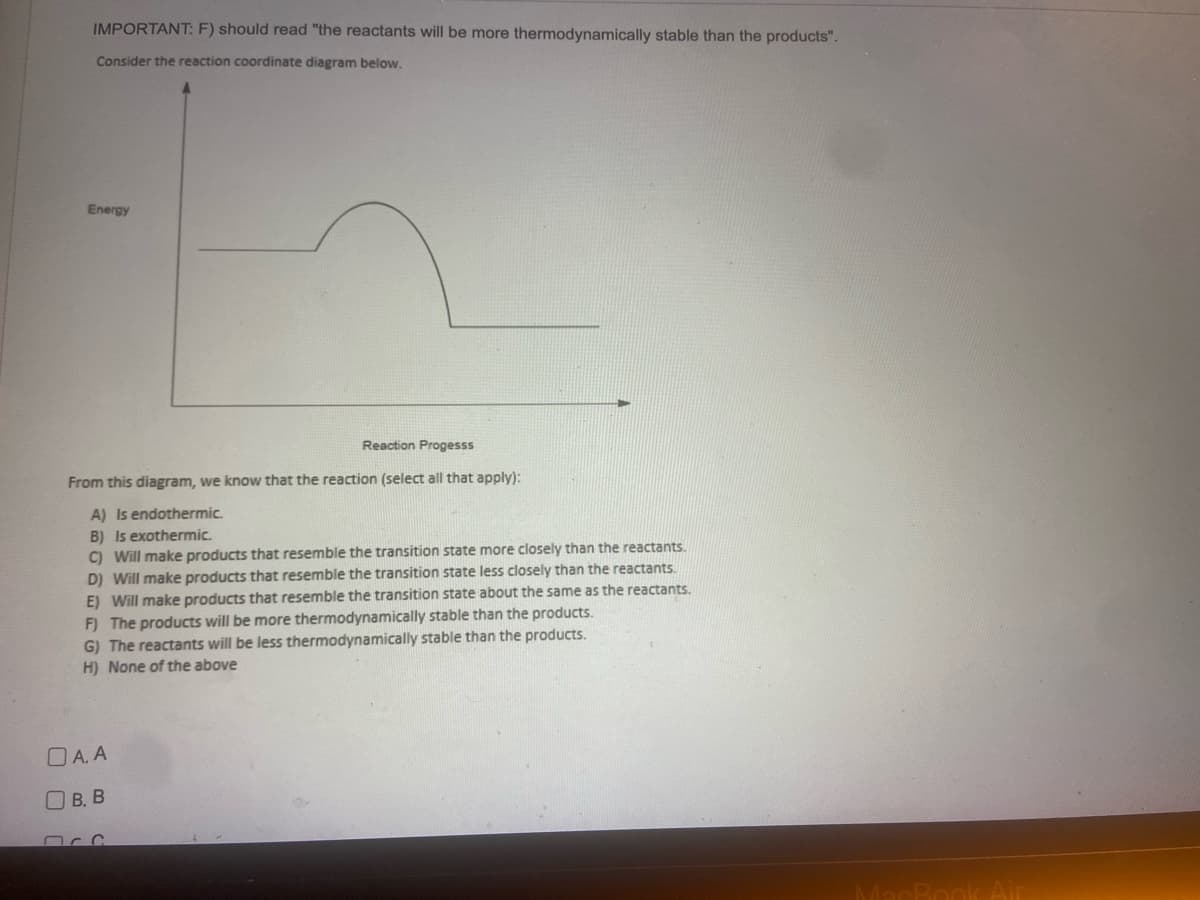
Transcribed Image Text:IMPORTANT: F) should read "the reactants will be more thermodynamically stable than the products".
Consider the reaction coordinate diagram below.
Energy
Reaction Progesss
From this diagram, we know that the reaction (select all that apply):
A) Is endothermic.
B) Is exothermic.
C) Will make products that resemble the transition state more closely than the reactants.
D) Will make products that resemble the transition state less closely than the reactants.
E) Will make products that resemble the transition state about the same as the reactants.
F) The products will be more thermodynamically stable than the products.
G) The reactants will be less thermodynamically stable than the products.
H) None of the above
DA. A
B. B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning