g. Write a balanced equation for the reaction that takes place between the organic product from part f and NaOH(aq). h. Draw the name of the products that form when the acid from part a is heated in the presence of (CH3CH2)2NH. i. Write a balanced equation for the reaction that takes place when the organic product from part h is heated in the presence of H2O and H+.
g. Write a balanced equation for the reaction that takes place between the organic product from part f and NaOH(aq). h. Draw the name of the products that form when the acid from part a is heated in the presence of (CH3CH2)2NH. i. Write a balanced equation for the reaction that takes place when the organic product from part h is heated in the presence of H2O and H+.
Chapter9: Alkynes: An Introduction To Organic Synthesis
Section9.SE: Something Extra
Problem 32AP
Related questions
Question
g. Write a balanced equation for the reaction that takes place between the organic product from part f and NaOH(aq).
h. Draw the name of the products that form when the acid from part a is heated in the presence of (CH3CH2)2NH.
i. Write a balanced equation for the reaction that takes place when the organic product from part h is heated in the presence of H2O and H+.

Transcribed Image Text:8.182: ma
al Which aad in +able 8.6 is the Strongest? n
Ka%3Dacid di sSociation constant
PKA = -109ka
gylocic acid Pka = 3.83
lactic acid PKa = 3.08 -1ess
Lactic acid 'is the Strongest
bo Name the, Conjugate base Ofthis acid
CH3-CH-C-OH
ட வற
ban
%3D
!!
%3D
CH2-CH-E
-
-0°:conjugate base
C Write the egUation for the reaction that takes place
between this acid and water.
CHs-CH-C - OH + HzD Z CHz-CH- C -0©+ H30+
一
ds Which isthere more of at eauilibrium,the acid in
your answeraor Hs conjugate base?
L'actic acid is Strongeř ačid than WCHE.
H2D Can easily accept H+ from the lactic acid and
from CH3-CH-E-C
00
base is present in more
amount.
~At equilibrium: conjugate
ea)oraw and name the sat that forms when the
acid from part a reacts wth KOHO
CH3-CH-C-0-H + KOH > CH3-CH-C-0°K+ +HzO
Base
Acid
SaH
Potassium
Lactate
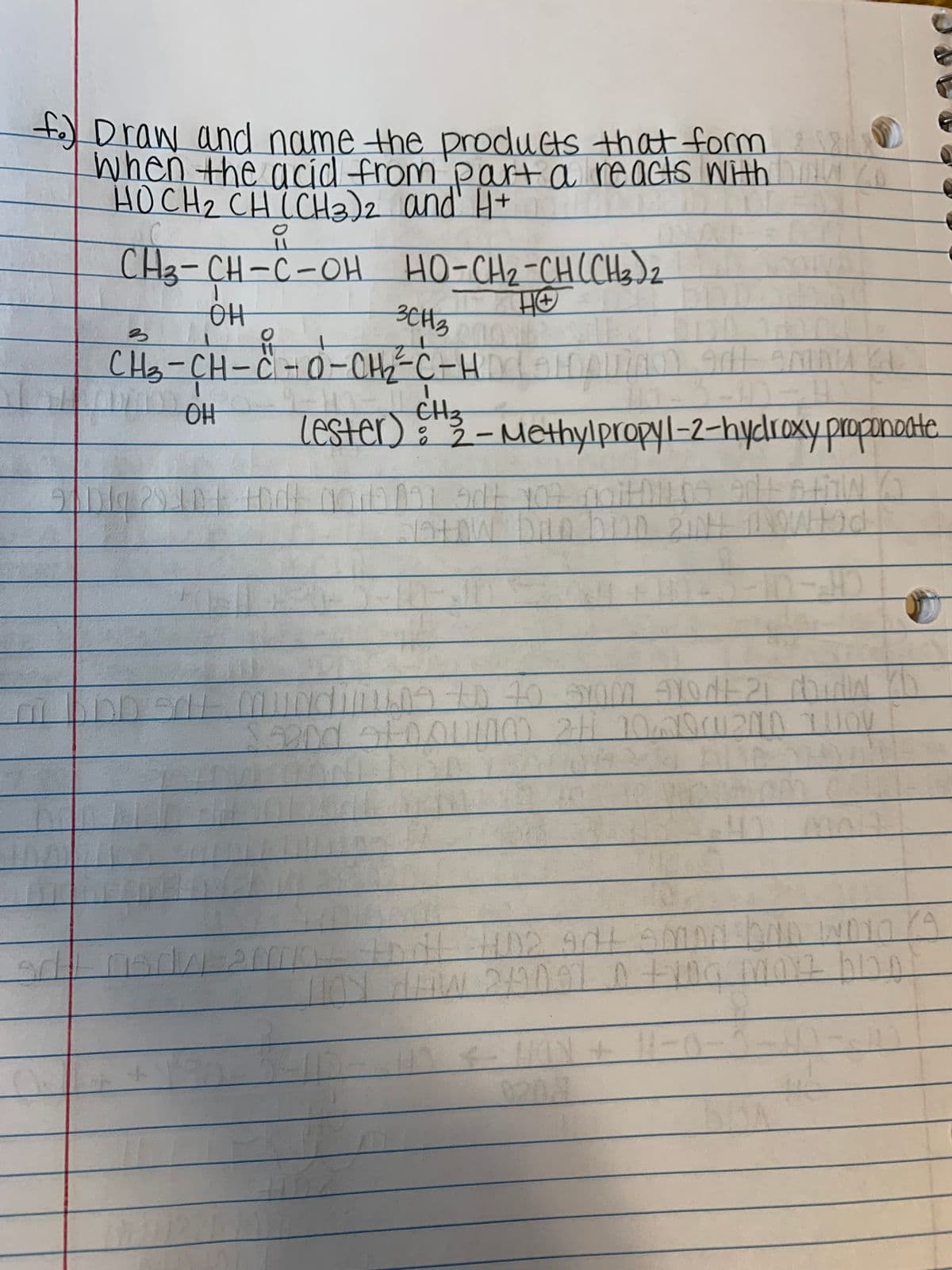
Transcribed Image Text:t Draw and name the products that form
when the acíd from'parta reacts Wth ih
HOCH, CH(CH)2 and' H+
요
CH3-CH-C-OH HO-CHz-CHCH)z
AA
CH3-CH-C-0-CH-C-H
CH3
LeSter) "3- Methylpropyl-2-hydroxyY proponante
211
0203
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning