Give the number of electrons for each set of quantum numbers: Number of electrons Quantum numbers ex n= 4; ml =-1 |means that all the ml that is equal to -1 that can be found in n=4 6 e 1 n= 2; 1= 1 n = 4; ml = 0; ms = ½ %3D 3 n= 1; ml = 0 2.
Give the number of electrons for each set of quantum numbers: Number of electrons Quantum numbers ex n= 4; ml =-1 |means that all the ml that is equal to -1 that can be found in n=4 6 e 1 n= 2; 1= 1 n = 4; ml = 0; ms = ½ %3D 3 n= 1; ml = 0 2.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter7: Quantum Theory Of The Atom
Section: Chapter Questions
Problem 7.32QP: Imagine a world in which the rule for the l quantum number is that values start with 1 and go up to...
Related questions
Question
Can you also help me with this worksheet??? Sorry for the inconvenience, I was not feeling these past days that's why I didn't attend the class, and I was left out.
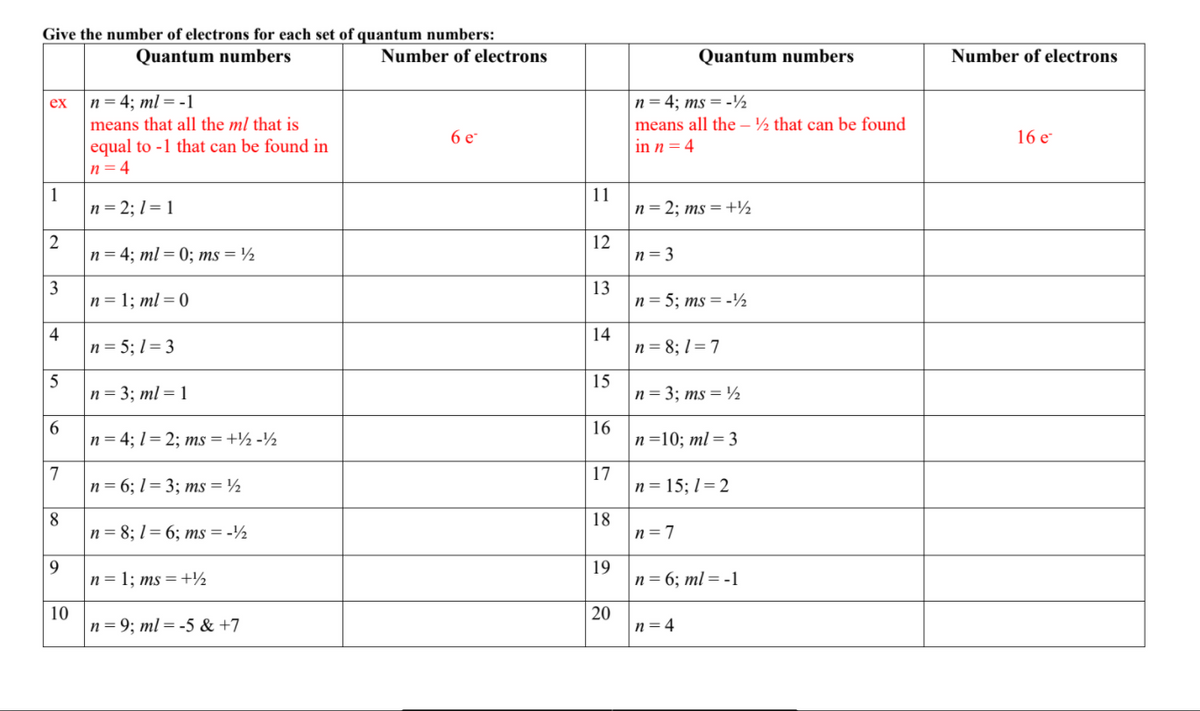
Transcribed Image Text:Give the number of electrons for each set of quantum numbers:
Quantum numbers
Number of electrons
Quantum numbers
Number of electrons
n = 4; ml = -1
means that all the ml that is
equal to -1 that can be found in
n = 4
n= 4; ms = -½
means all the – ½ that can be found
ex
6 e-
16 e
in n = 4
1
n= 2; 1= 1
11
n = 2; ms =+½
2
n = 4; ml = 0; ms = ½
n = 3
3
n= 1; ml = 0
13
n= 5; ms = -½
4
n= 5; 1= 3
14
n = 8; 1 = 7
n= 3; ml = 1
15
|n= 3; ms = ½
16
6.
n = 4; 1 = 2; ms =+½ -½
n =10; ml = 3
7
n= 6; 1= 3; ms = ½
17
n= 15; 1= 2
8
n= 8; 1 = 6; ms = -½
18
n=7
9
n = 1; ms =+½
19
n= 6; ml = -1
10
n = 9; ml = -5 & +7
20
n= 4
12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning