Given 100 mL of a solution that contains 0.80 mM Ag* and 0.80 mM Cu*, can the two metals be separated by selective precipitation as the insoluble bromide salts by adding 100 mL of an 8.0 mM solution of KBr? Ksp values are 6.27 x 10-9 for CuBr and 5.35 x 10 13 for AgBr. Yes, the two metals can be separated. No, the two metals cannot be separated.
Given 100 mL of a solution that contains 0.80 mM Ag* and 0.80 mM Cu*, can the two metals be separated by selective precipitation as the insoluble bromide salts by adding 100 mL of an 8.0 mM solution of KBr? Ksp values are 6.27 x 10-9 for CuBr and 5.35 x 10 13 for AgBr. Yes, the two metals can be separated. No, the two metals cannot be separated.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.24QAP
Related questions
Question
Transcribed Image Text:Full Screen
O Accessibility
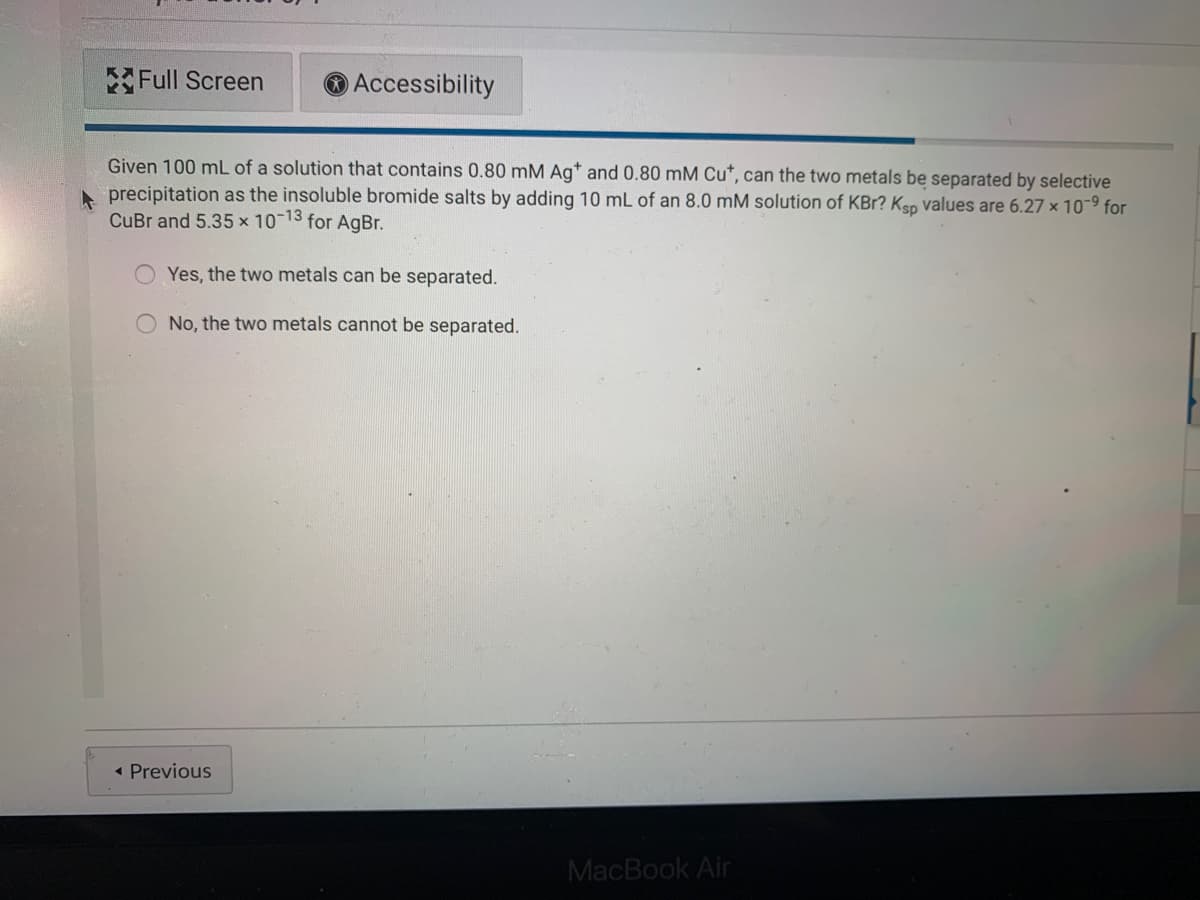
Given 100 mL of a solution that contains 0.80 mM Ag* and 0.80 mM Cu*, can the two metals be separated by selective
precipitation as the insoluble bromide salts by adding 10 mL of an 8.0 mM solution of KBr? Kso values are 6.27 x 10-9 for
CuBr and 5.35 x 10-13 for AgBr.
Yes, the two metals can be separated.
No, the two metals cannot be separated.
« Previous
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax