Given the following molecules, identify the polarity of the molecules involved and the type of intermolecular force/s (London Dispersion Forces, Dipole-Dipole Forces, Hydrogen Bonding, and lon-Dipole Forces) present. You can write more than one type of intermolecular force per item.
Given the following molecules, identify the polarity of the molecules involved and the type of intermolecular force/s (London Dispersion Forces, Dipole-Dipole Forces, Hydrogen Bonding, and lon-Dipole Forces) present. You can write more than one type of intermolecular force per item.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.37PAE: 8.37 If a molecule is not very polarizable, how will it respond to an external electric field?
Related questions
Question
Given the following molecules, identify the polarity of the molecules involved and the type of intermolecular force/s (London Dispersion Forces, Dipole-Dipole Forces, Hydrogen Bonding, and lon-Dipole Forces) present. You can write more than one type of intermolecular force per item.
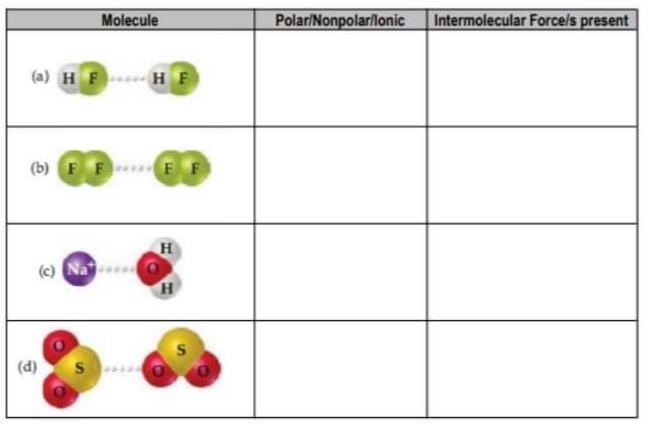
Transcribed Image Text:Molecule
PolariNonpolar/lonic
Intermolecular Forcels present
(a) HF
HF
(b) FF
FF
(c) Na
(d)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning