positions in a ccp/fcc lattice of copper. a. Prove mathematically that identical atoms in a ccp/fcc structure occupy 74.0% of the space when considered as hard spheres of radius r. 4 Volume of a sphere = V =Tr³ 3 a b. Use the metallic radius of copper (128 pm) and its atomic weight (63.456 amu) to calculate the density of pure copper metal in g/cm³. Given that the atomic weight of tin is 118.71 amu, and assuming that its metallic radius is identical to that of copper in the alloy, calculate the density of speculum metal. С.
positions in a ccp/fcc lattice of copper. a. Prove mathematically that identical atoms in a ccp/fcc structure occupy 74.0% of the space when considered as hard spheres of radius r. 4 Volume of a sphere = V =Tr³ 3 a b. Use the metallic radius of copper (128 pm) and its atomic weight (63.456 amu) to calculate the density of pure copper metal in g/cm³. Given that the atomic weight of tin is 118.71 amu, and assuming that its metallic radius is identical to that of copper in the alloy, calculate the density of speculum metal. С.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter11: Liquids And Solids
Section: Chapter Questions
Problem 11.87QE
Related questions
Question
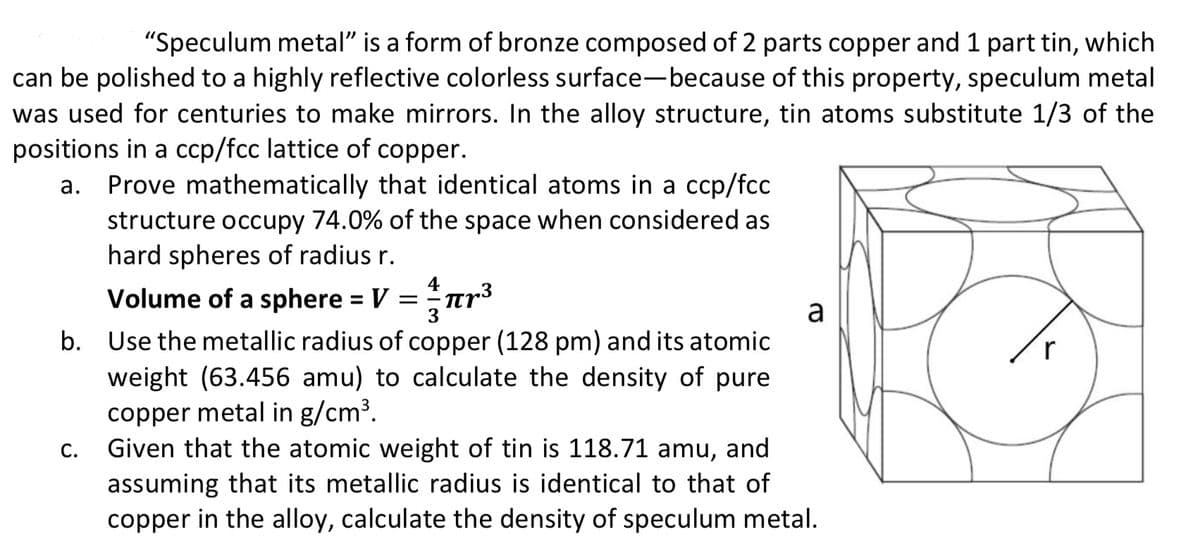
Transcribed Image Text:"Speculum metal" is a form of bronze composed of 2 parts copper and 1 part tin, which
can be polished to a highly reflective colorless surface-because of this property, speculum metal
was used for centuries to make mirrors. In the alloy structure, tin atoms substitute 1/3 of the
positions in a ccp/fcc lattice of copper.
Prove mathematically that identical atoms in a ccp/fcc
structure occupy 74.0% of the space when considered as
hard spheres of radius r.
Volume of a sphere = V =nr3
%3D
a
b. Use the metallic radius of copper (128 pm) and its atomic
weight (63.456 amu) to calculate the density of pure
copper metal in g/cm³.
Given that the atomic weight of tin is 118.71 amu, and
assuming that its metallic radius is identical to that of
copper in the alloy, calculate the density of speculum metal.
С.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,