Given the following reactions: Anode: NO3- + 2H+ + e- --> NO2 + H2O Cathode: 2H+ + 2e- - --> H2 Redox: 2NO2(g) + 2 H2O (I) --> 2NO3-(aq) + 2H+(aq) + H2(g) Conditions: [0.15 atm] [0.18 atm] [0.1 M] [0.1 M] [0.67 atm] Find the cell potential, E Report your answer in 2 decimal places. Do not include the unit
Given the following reactions: Anode: NO3- + 2H+ + e- --> NO2 + H2O Cathode: 2H+ + 2e- - --> H2 Redox: 2NO2(g) + 2 H2O (I) --> 2NO3-(aq) + 2H+(aq) + H2(g) Conditions: [0.15 atm] [0.18 atm] [0.1 M] [0.1 M] [0.67 atm] Find the cell potential, E Report your answer in 2 decimal places. Do not include the unit
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 150CP: Given the following two standard reduction potentials, solve for the standard reduction potential of...
Related questions
Question
![Given the following reactions:
Anode: NO3- + 2H+ + e-
-> NO2 +
H2O
Cathode: 2H+ + 2e-
--> H2
Redox:
2NO2(g) + 2
H2O (I) --> 2NO3-(aq) + 2H+(aq) +
H2(g)
Conditions:
[0.15 atm]
[0.18 atm]
[0.1 M]
[0.1 M]
[0.67 atm]
Find the cell potential, E
Report your answer in 2 decimal
places. Do not include the unit](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff89e3c2e-507d-408b-bcde-00a189798902%2F0e20ac20-d024-4050-a69c-6db75eb7b794%2F7vfgiqp_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Given the following reactions:
Anode: NO3- + 2H+ + e-
-> NO2 +
H2O
Cathode: 2H+ + 2e-
--> H2
Redox:
2NO2(g) + 2
H2O (I) --> 2NO3-(aq) + 2H+(aq) +
H2(g)
Conditions:
[0.15 atm]
[0.18 atm]
[0.1 M]
[0.1 M]
[0.67 atm]
Find the cell potential, E
Report your answer in 2 decimal
places. Do not include the unit
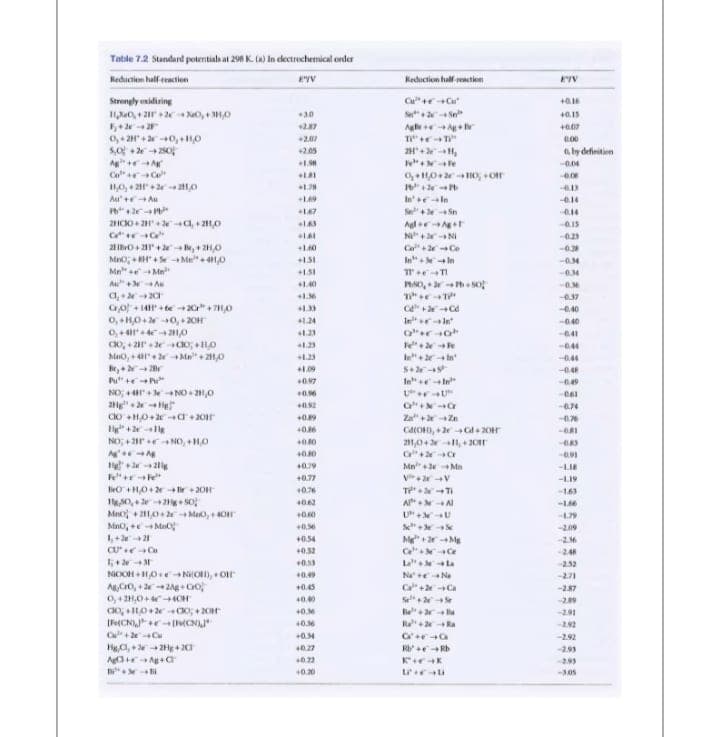
Transcribed Image Text:Table 7.2 Standard potentialh at 29 K. a) in dectrechenicat order
Reduction half eaction
Rediction half-eactien
Strengly onidiring
Cu"+ Cu
30
+0.15
Ag v
0,+ 2H+20, ,0
5,0 +220
+207
+2.05
2H+ ,
aby definitien
Co"r C
Au' + Au
Ineln
Se+ Sn
2HCO+ + 4,0
-0.15
N+ NI
1.60
Co"+ Ce
MnO; H+Se Me"+ 10
Mn" eMe
Au+ A
In In
1.40
0.37
Co + 14r +te +20 +70
0,+H0+0, + 20H
0, + 21,0
C + Cd
0.40
+1.24
Inrln
-040
1.23
044
Mo,+ + Ma+ 21,0
0.44
S+
In In
1.09
-0.4
P" te
097
49
o'u ON +anoN
Za"+ Zn
40.6
BRI
NO; +2rr NO, +HO
211,0+ 1,+ 20
+0.10
40.79
Mn" +2Mn
--LIE
+0.77
-L19
tO +H0+2 -fir + 20
0,+ 2g+ So
Mno + 10+ Mao, 4O
+0.76
16)
A NA
179
S+
209
1,+ 2e+21
+0.54
Ce Ce
L la
+0.52
24
+
NioOH + H0e NiO), + Or
ACro, + 2e2Ag Co
0,+2H,0+ 4CH
0.53
252
Na e Na
-271
+0.45
Ca+ Ca
-287
40.00
Se
-2.39
40.
-2.91
40.
R a
292
+0.34
-2.92
Ha,+ZHg +2
40.27
-2.99
0.22
-2.93
+0.20
--AOS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning