Given the thermodynamic data in the table below, calculate the equlibirum constant (at 298 K) for the reaction below: 2502 (g) + 02 (g) 2503 (g) Substance AH°F (k) / mol) S° U/ mol-K) SO2 (g) 249 -297 205 02 (g) S03 (g) -395 256 a) calculate the AH for the reaction using Hess's Law and the data above (report your units): AHn=EnxH products 2mx Hi reactants b) calculate the AS for the reaction using the data above (report your units) ASPxn=EnxS products 2mx S reactants C) calculate the AG for the reaction at 25 °C (298 K) (again pay attention to your units) (report your answer in kJ {1 kJ = 1000 J})
Given the thermodynamic data in the table below, calculate the equlibirum constant (at 298 K) for the reaction below: 2502 (g) + 02 (g) 2503 (g) Substance AH°F (k) / mol) S° U/ mol-K) SO2 (g) 249 -297 205 02 (g) S03 (g) -395 256 a) calculate the AH for the reaction using Hess's Law and the data above (report your units): AHn=EnxH products 2mx Hi reactants b) calculate the AS for the reaction using the data above (report your units) ASPxn=EnxS products 2mx S reactants C) calculate the AG for the reaction at 25 °C (298 K) (again pay attention to your units) (report your answer in kJ {1 kJ = 1000 J})
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section: Chapter Questions
Problem 87SCQ: The Haber-Bosch process for the production of ammonia is one of the key industrial processes in...
Related questions
Question
Please answer a,b,c, and d!!
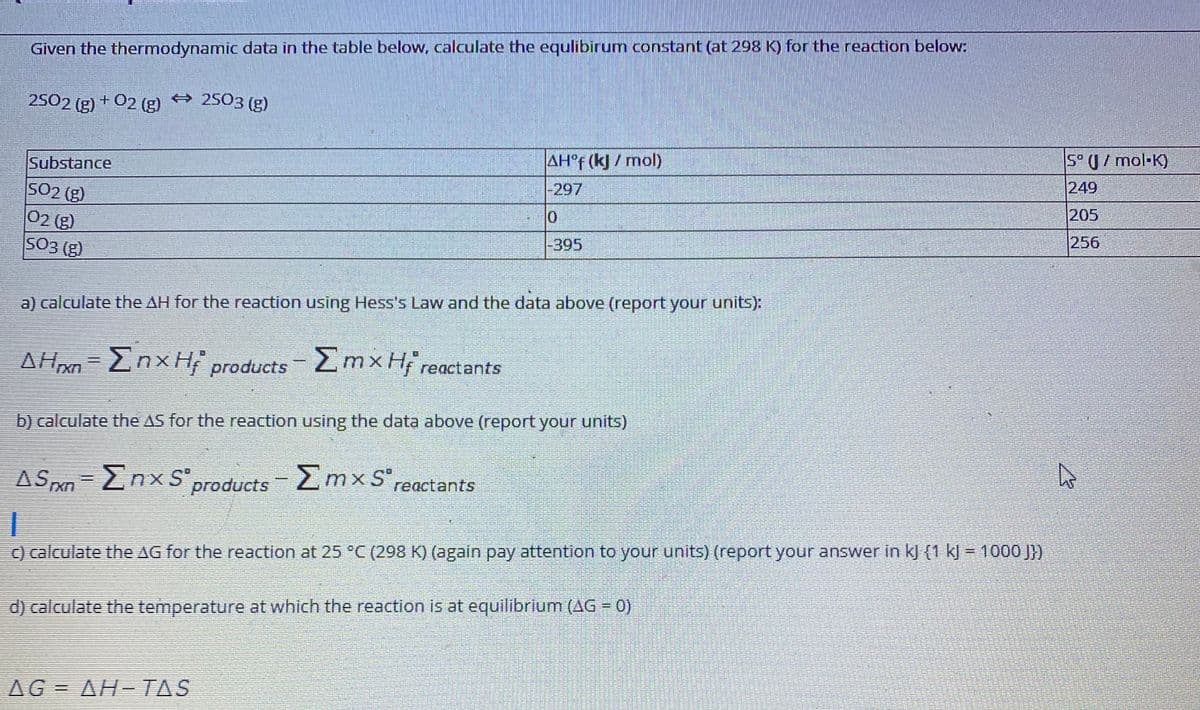
Transcribed Image Text:Given the thermodynamic data in the table below, calculate the equlibirum constant (at 298 K) for the reaction below:
2502 (g) + 02 (g) 2503 (g)
5° U/ mol-K)
AH°F (k)/ mol)
Substance
249
-297
SO2 (g)
02 (g)
SO3 (g)
205
256
-395
a) calculate the AH for the reaction using Hess's Law and the data above (report your units):
Znx H products 2mxH; reactants
b) calculate the AS for the reaction using the data above (report your units)
AS xn-EnxSproducts mxS",
Σmxs
reactants
) calculate the AG for the reaction at 25 °C (298 K) (again pay attention to your units) (report your answer in kj (1 kJ = 1000 J})
d) calculate the temperature at which the reaction is at equilibrium (AG = 0)
AG = AH-TAS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax