Glucuronic acid is an oxidized derivative of glucose found in animal secretions such as saliva. Biological conversion of glucuronic acid into its isomer, fructuronic acid, is catalyzed by an enzyme called uronic acid isomerase. OH HO HOʻ H20, H* HỌ HO Но ОН ОН uronic acid isomerase HO ОН fructuronic acid glucuronic acid Provide a complete curved-arrow mechanism for this transformation. You may assume that the enzyme active site will supply all necessary equivalents of water and acid in order to accomplish the reaction. (In other words, draw the mechanism as if the only reagents were H3O* and H20.)
Glucuronic acid is an oxidized derivative of glucose found in animal secretions such as saliva. Biological conversion of glucuronic acid into its isomer, fructuronic acid, is catalyzed by an enzyme called uronic acid isomerase. OH HO HOʻ H20, H* HỌ HO Но ОН ОН uronic acid isomerase HO ОН fructuronic acid glucuronic acid Provide a complete curved-arrow mechanism for this transformation. You may assume that the enzyme active site will supply all necessary equivalents of water and acid in order to accomplish the reaction. (In other words, draw the mechanism as if the only reagents were H3O* and H20.)
Chapter17: Alcohols And Phenols
Section17.SE: Something Extra
Problem 73AP
Related questions
Question
Can you help me understand how to approach this question and explain the mechanism?
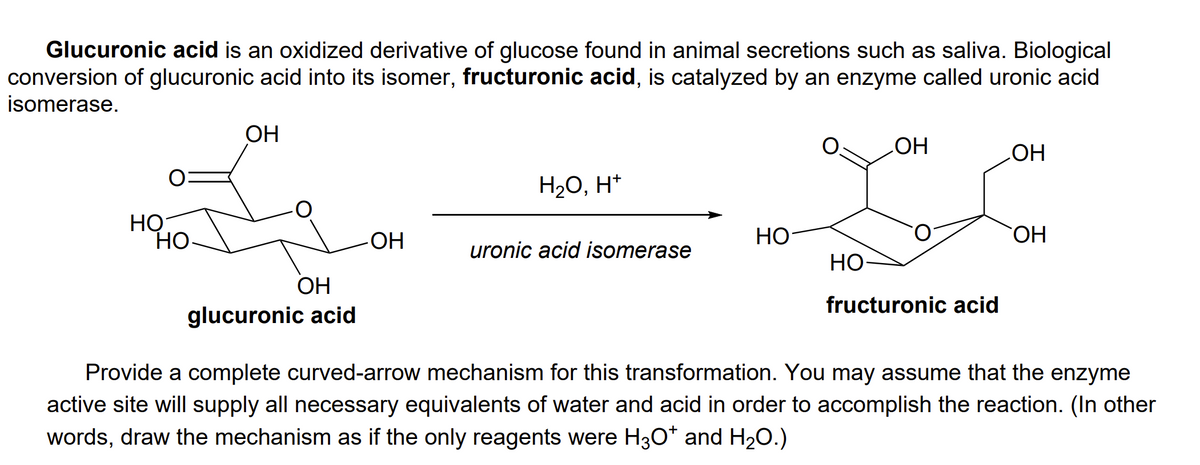
Transcribed Image Text:Glucuronic acid is an oxidized derivative of glucose found in animal secretions such as saliva. Biological
conversion of glucuronic acid into its isomer, fructuronic acid, is catalyzed by an enzyme called uronic acid
isomerase.
OH
HO
HO
H20, H*
НО
Но-
Но-
НО
HO
uronic acid isomerase
Но
ОН
fructuronic acid
glucuronic acid
Provide a complete curved-arrow mechanism for this transformation. You may assume that the enzyme
active site will supply all necessary equivalents of water and acid in order to accomplish the reaction. (In other
words, draw the mechanism as if the only reagents were H3O* and H20.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning