Glycerol has many consumer uses. It is found in many beauty products including moisturizing soaps. Due to its sweet taste and non-toxicity, it is also found in sugar-free desserts. Which intermolecular force is primarily associated with a sample of pure glycerol? ОН HO OH hydrogen bonding ionic forces dispersion forces dipole forces
Glycerol has many consumer uses. It is found in many beauty products including moisturizing soaps. Due to its sweet taste and non-toxicity, it is also found in sugar-free desserts. Which intermolecular force is primarily associated with a sample of pure glycerol? ОН HO OH hydrogen bonding ionic forces dispersion forces dipole forces
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 55SCQ
Related questions
Question
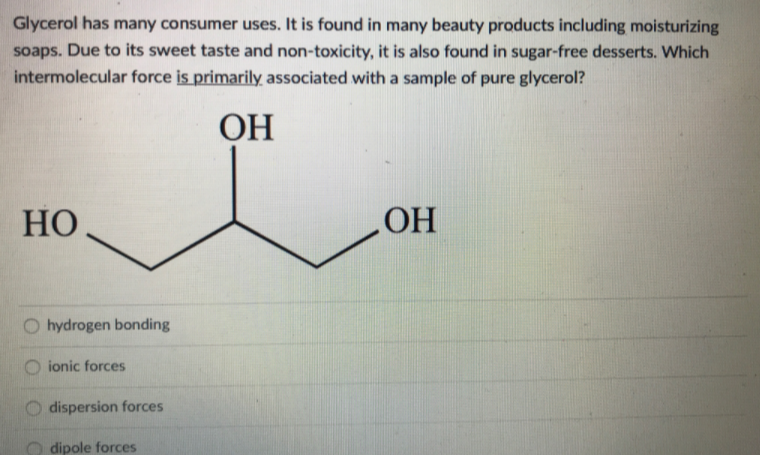
Transcribed Image Text:Glycerol has many consumer uses. It is found in many beauty products including moisturizing
soaps. Due to its sweet taste and non-toxicity, it is also found in sugar-free desserts. Which
intermolecular force is primarily associated with a sample of pure glycerol?
ОН
HO
OH
hydrogen bonding
ionic forces
dispersion forces
dipole forces
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning