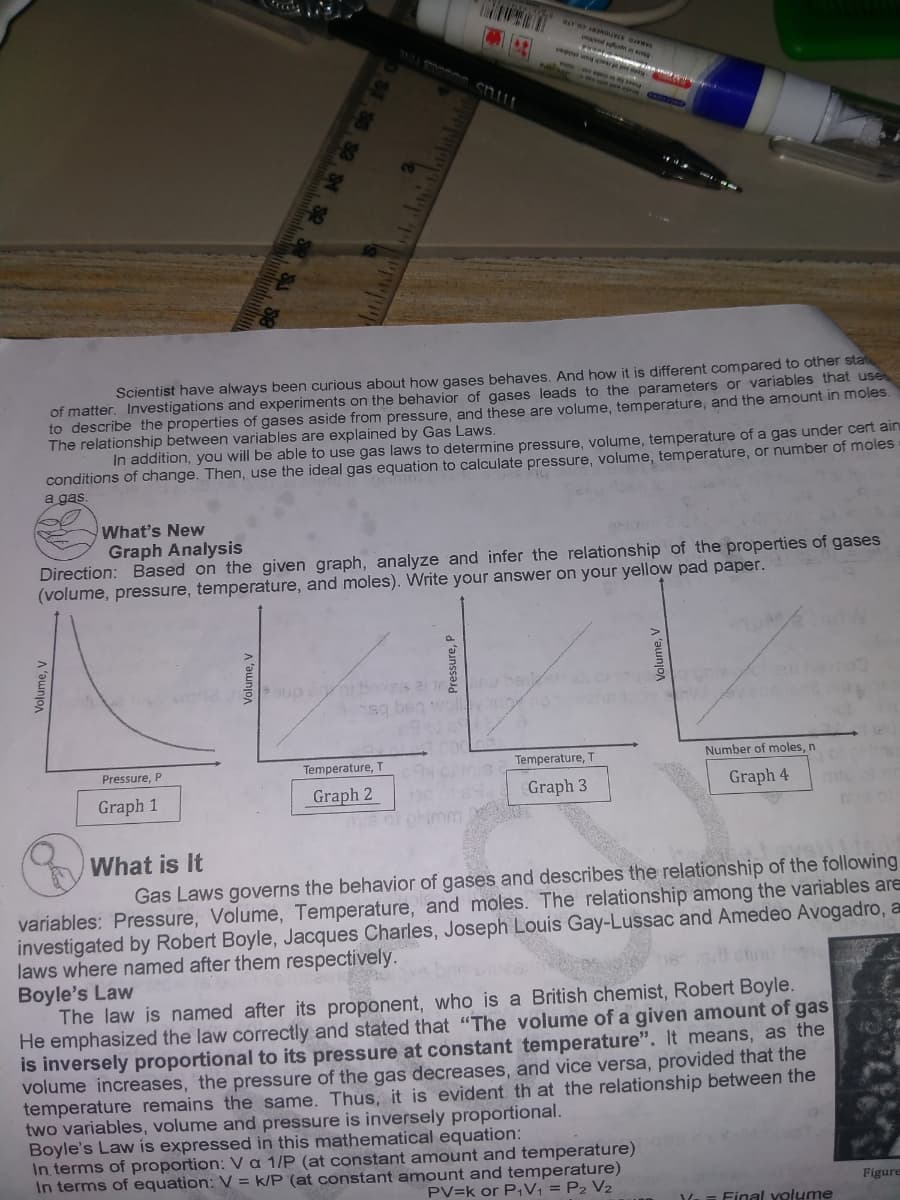
Graph Analysis Direction: Based on the given graph, analyze and infer the relationship of the properties of gases (volume, pressure, temperature, and moles). Write your answer on your yellow pad paper. Pressure, P Temperature, T Temperature, T Number of moles, n Graph 1 Graph 2 Graph 3 Graph 4 Volume, V Volume, V Pressure, P Volume, V
Graph Analysis Direction: Based on the given graph, analyze and infer the relationship of the properties of gases (volume, pressure, temperature, and moles). Write your answer on your yellow pad paper. Pressure, P Temperature, T Temperature, T Number of moles, n Graph 1 Graph 2 Graph 3 Graph 4 Volume, V Volume, V Pressure, P Volume, V
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 31Q
Related questions
Question
100%
based on the graph analyze and infer the relationship between the properties of gases
Transcribed Image Text:g yo
STATIO
u d m ag.
VAMAYO ONERY CO.ATD
Scientist have always been curious about how gases behaves, And how it is different compared to other stat
of matter. Investigations and experiments on the behavior of gases leads to the parameters or variables that use
to describe the properties of gases aside from pressure, and these are volume, temperature, and the amount in moles.
The relationship between variables are explained by Gas Laws.
In addition, you will be able to use gas laws to determine pressure, volume, temperature of a gas under cert ain
conditions of change. Then, use the ideal gas equation to calculate pressure, volume, temperature, or number of moles
a gas.
What's New
Graph Analysis
Direction: Based on the given graph, analyze and infer the relationship of the properties of gases
(volume, pressure, temperature, and moles). Write your answer on your yellow pad paper.
up baes
sg beg wol
10s Temperature, T
Graph 3
Pressure, P
Temperature, T
Number of moles, n
Graph 1
Graph 2
Graph 4
mm
What is It
Gas Laws governs the behavior of gases and describes the relationship of the following
variables: Pressure, Volume, Temperature, and moles. The relationship among the variables are
investigated by Robert Boyle, Jacques Charles, Joseph Louis Gay-Lussac and Amedeo Avogadro, a
laws where named after them respectively.
Boyle's Law
The law is named after its proponent, who is a British chemist, Robert Boyle.
He emphasized the law correctly and stated that "The volume of a given amount of gas
is inversely proportional to its pressure at constant temperature". It means, as the
volume increases, the pressure of the gas decreases, and vice versa, provided that the
temperature remains the same. Thus, it is evident th at the relationship between the
two variables, volume and pressure is inversely proportional.
Boyle's Law is expressed in this mathematical equation:
In terms of proportion: Va 1/P (at constant amount and temperature)
In terms of equation: V = k/P (at constant amount and temperature)
PV=k or P,V; = P2 V2
Figure
Y = Final volume
Volume, V
Pressure, P
Volume, V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning