Data Table 3: Temperature (K) Volume (L) Point 1 298 1.00 Point 2 596 2.00 Point 3 447 1.50 Point 4 745 2.50 Point 5 864 2.90 Based on the data collected, make a statement about the trend that can be observed between the temperature and pressure of a gas. Copy Data Table 3 into Excel. Use Excel to create a graph with Pressure (in atm) on the horizontal axis (the independent variable) and Temperature (in kelvins) on the vertical axis (the dependent variable). Set the scale on each of the axes to show O K and O atm. Draw the best straight line that goes through most of the points and extrapolate (extend) the line until it goes through O atm. Does the graph show that Pis proportional to Tas stated by Amonton's and Gay-Lussac's Law? Absolute zero is O K. What is the temperature reading when the extrapolated line goes through 0 atm? Considering what is now known about Amonton's and Gay-Lussac's Law, make a prediction based on the following situation: What would happen to the pressure of a gas inside a sealed bottle, if the bottle was cooled fo half of its original temperature?
Data Table 3: Temperature (K) Volume (L) Point 1 298 1.00 Point 2 596 2.00 Point 3 447 1.50 Point 4 745 2.50 Point 5 864 2.90 Based on the data collected, make a statement about the trend that can be observed between the temperature and pressure of a gas. Copy Data Table 3 into Excel. Use Excel to create a graph with Pressure (in atm) on the horizontal axis (the independent variable) and Temperature (in kelvins) on the vertical axis (the dependent variable). Set the scale on each of the axes to show O K and O atm. Draw the best straight line that goes through most of the points and extrapolate (extend) the line until it goes through O atm. Does the graph show that Pis proportional to Tas stated by Amonton's and Gay-Lussac's Law? Absolute zero is O K. What is the temperature reading when the extrapolated line goes through 0 atm? Considering what is now known about Amonton's and Gay-Lussac's Law, make a prediction based on the following situation: What would happen to the pressure of a gas inside a sealed bottle, if the bottle was cooled fo half of its original temperature?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
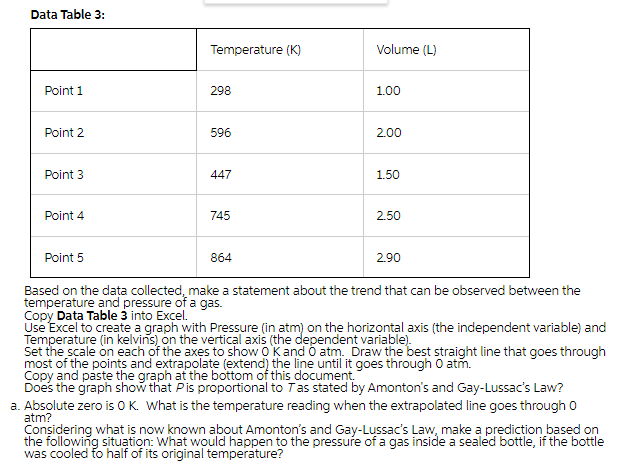
Transcribed Image Text:Data Table 3:
Temperature (K)
Volume (L)
Point 1
298
1.00
Point 2
596
2.00
Point 3
447
1.50
Point 4
745
2.50
Point 5
864
2.90
Based on the data collected, make a statement about the trend that can be observed between the
temperature and pressure of a gas.
Copy Data Table 3 into Excel.
Use Excel to create a graph with Pressure (in atm) on the horizontal axis (the independent variable) and
Temperature (in kelvins) on the vertical axis (the dependent variable).
Set the scale on each of the axes to show O Kand O atm. Draw the best straight line that goes through
most of the points and extrapolate (extend) the line until it goes through O atm.
Copy and paste the graph at the bottom of this document.
Does the graph show that Pis proportional to Tas stated by Amonton's and Gay-Lussac's Law?
a. Absolute zero is OK. What is the temperature reading when the extrapolated line goes through o
atm?
Considering what is now known about Amonton's and Gay-Lussac's Law, make a prediction based on
the following situation: What would happen to the pressure of a gas inside a sealed bottle, if the bottle
was cooled fo half of its original temperature?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 7 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning