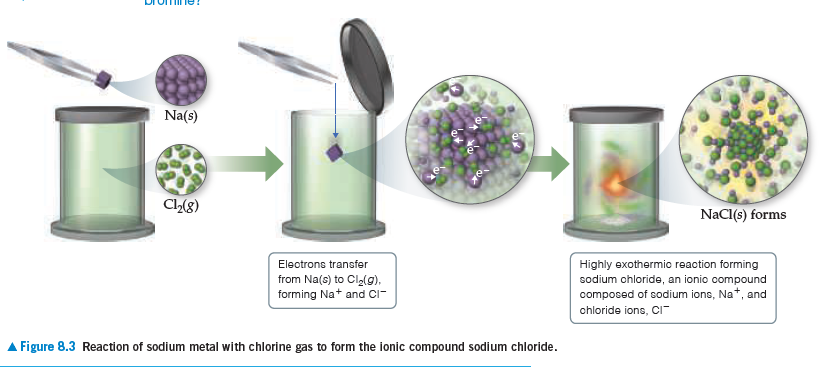
Group 1A Group 3A Grоцр 7А Слиир 2А Crоир бл ut 0.90 0.59 1.25 0.41 1.19 LI 1.25 a54 0.96 057 Na L16 Mg O86 1.70 1.67 Na 1.66 Mg 141 AI 1.21 1.05 1.02 к 1.52 Ca Ga a76 Br 1.2 1.14 1.84 Ca 1.76 Ga 1.22 Hr 1.20 1.20 2.00 кь 166 Te- 2.00 1- 2.06 a94 1.32 In 1.42 кь 2.20 Te 1.35 1.95 1.39 cation anion - neueral amm AFigure 7 Na(s) Cl2(8) NaCl(s) forms Electrons transfer Highly exothermic reaction forming sodium ohloride, an ionio compound composed of sodium ions, Na*, and from Na(6) to Cla(g), forming Na+ and CIr- chloride ions, CI Figure 8.3 Reaction of sodium metal with chlorine gas to form the ionic compound sodium chloride.
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
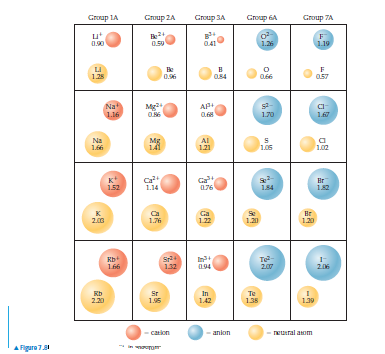
The ionic compound CaO crystallizes with the same structure
as sodium chloride (Figure 8.3). (a) In this structure,
how many O2- are in contact with each Ca2+ ion (Hint: Remember
the pattern of ions shown in Figure 8.3 repeats over
and over again in all three directions.) (b) Would energy be
consumed or released if a crystal of CaO was converted to
a collection of widely separated Ca—O ion pairs? (c) From
the ionic radii given in Figure 7.8, calculate the potential
energy of a single Ca—O ion pair that is just touching
(the magnitude of electronic charge is given on the inside
back cover). (d) Calculate the energy of a mole of such pairs.
How does this compare to the lattice energy of CaO? (e)
What factor do you think accounts for most of the discrepancy
between the energies in part (d)—the bonding in CaO
is more covalent than ionic, or the electrostatic interactions
in a crystal lattice are more complicated than those in a single
ion pair?
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images







