guestion 29 The OH radical reacts with itself in a disproportionation type reaction according to the reaction: OH + OH H20 + 0 This rate constant at room temperature is 3.81 x 10 M1s1. If the initial concentration of OH is 2.14 x 10-7 M, what is the first half life for the reaction (in seconds)? (the answer should be entered with 3 significant figures; do not enter units; give answer in scientific notation–valid notation examples include 1.23e-8 and 1.23e8 and -1.23e-4 and 1.23e0)
guestion 29 The OH radical reacts with itself in a disproportionation type reaction according to the reaction: OH + OH H20 + 0 This rate constant at room temperature is 3.81 x 10 M1s1. If the initial concentration of OH is 2.14 x 10-7 M, what is the first half life for the reaction (in seconds)? (the answer should be entered with 3 significant figures; do not enter units; give answer in scientific notation–valid notation examples include 1.23e-8 and 1.23e8 and -1.23e-4 and 1.23e0)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 9QAP: Experimental data are listed for the following hypothetical reaction: A+Bproducts (a) Plot these...
Related questions
Question
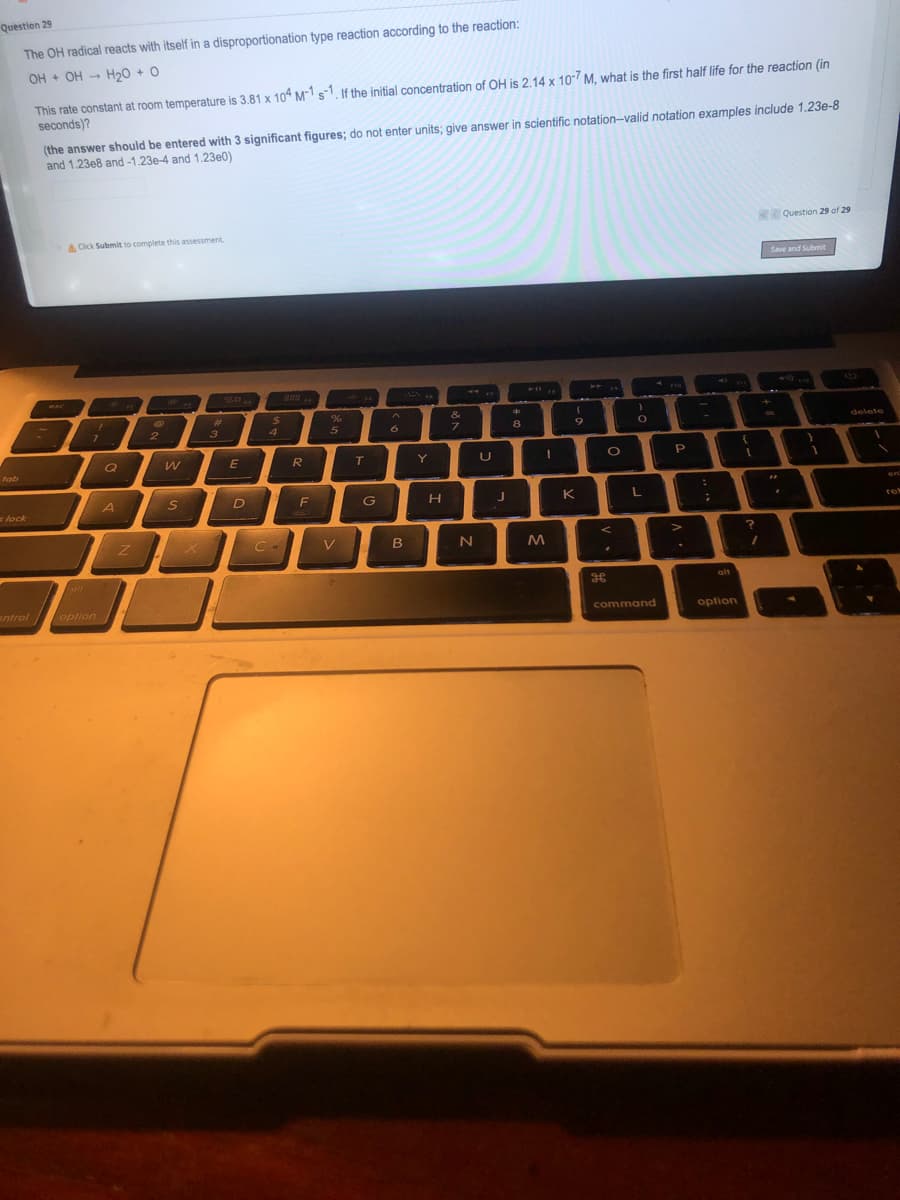
Transcribed Image Text:Question 29
The OH radical reacts with itself in a disproportionation type reaction according to the reaction:
OH + OH -
H20 + O
This rate constant at room temperature is 3.81 x 10 Ms1, If the initial concentration of OH is 2.14 x 10 M, what is the first half life for the reaction (in
seconds)?
(the answer should be entered with 3 significant figures; do not enter units; give answer in scientific notation-valid notation examples include 1.23e-8
and 1.23e8 and -1.23e-4 and 1.23e0)
Question 29 of 29
A Click Submit to complete this assessment.
Save and Submit
24
PI1
* 44
%23
&
3
4
5
9
delete
Q
R
T
Y
U
P
tab
en
lock
A
S
D
F
G
K
L
V
all
alt
antrol
option
command
option
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning