Draw both pyranose anomers of each aldohexose using a threedimensional
representation with a chair pyranose. Label each anomer as
α or β.
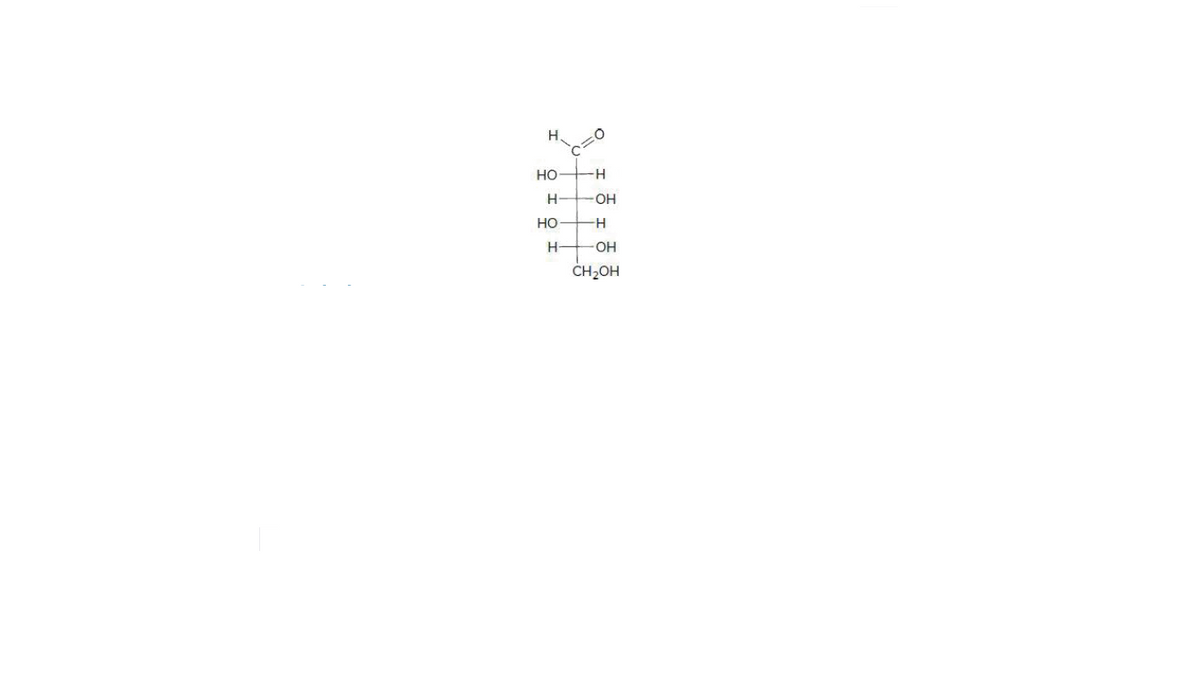
The given structure is,
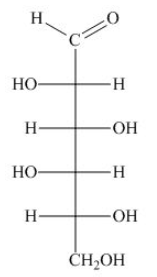
The Fischer projection of D-sugar is converted to Haworth projection.
In step 1, The formation of pyranose ring takes place by the attack of hydroxyl substituent at carbon fifth on carbonyl carbon.
In step 2, is drawn above the ring in Haworth projection of D-pyranose.
In step 3, At carbon first position, the substituents and are cis to one other in beta-form. So, they are drawn above the ring. The substituents and are trans to one other in alpha-form. So, substituent is drawn below the ring.
In step 4, the substituents that are found in right side in Fischer projection are drawn below the ring in Haworth projection. The substituents that are found in left side in Fischer projection are drawn above ring in Haworth projection.
Step by step
Solved in 4 steps with 5 images


