H2 O2 Some time after Stopcock closed Stopcock open Stopcock open (a) (b) (C) Figure 9.27 (a) Two gases, H2 and 02, are initially separated. (b) When the stopcock is opened, they mix together. The lighter gas, H2, passes through the opening faster than O2, so just after the stopcock is opened, more H2 molecules move to the O2 side than O2 molecules move to the H2 side. (c) After a short time, both the slower-moving Oz molecules and the faster-moving H2 molecules have distributed themselves evenly on both sides of the vessel. 예
H2 O2 Some time after Stopcock closed Stopcock open Stopcock open (a) (b) (C) Figure 9.27 (a) Two gases, H2 and 02, are initially separated. (b) When the stopcock is opened, they mix together. The lighter gas, H2, passes through the opening faster than O2, so just after the stopcock is opened, more H2 molecules move to the O2 side than O2 molecules move to the H2 side. (c) After a short time, both the slower-moving Oz molecules and the faster-moving H2 molecules have distributed themselves evenly on both sides of the vessel. 예
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 8ALQ
Related questions
Question
Explain why the numbers of molecules are not identical in the left- and right-hand bulbs shown in the center illustration as shown.
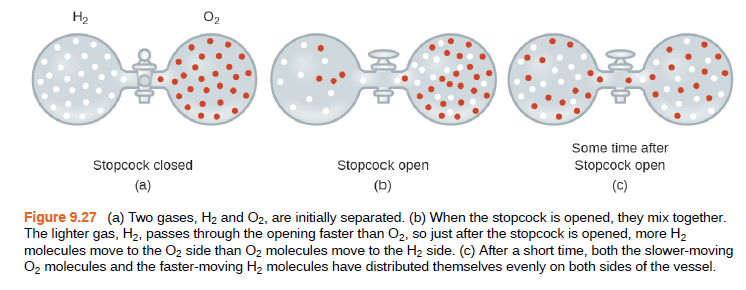
Transcribed Image Text:H2
O2
Some time after
Stopcock closed
Stopcock open
Stopcock open
(a)
(b)
(C)
Figure 9.27 (a) Two gases, H2 and 02, are initially separated. (b) When the stopcock is opened, they mix together.
The lighter gas, H2, passes through the opening faster than O2, so just after the stopcock is opened, more H2
molecules move to the O2 side than O2 molecules move to the H2 side. (c) After a short time, both the slower-moving
Oz molecules and the faster-moving H2 molecules have distributed themselves evenly on both sides of the vessel.
예
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning