H,CO A в H. D Proton NMR: 7.1 (doublet, 2H), 6.9 (doublet, 2H), 4.0 (quartet, 2H), 3.5 (singlet, 2H), 2.2 (singlet, 3H), 1.3 (triplet, 3H) Carbon-13 NMR: 205, 157, 130, 126, 114, 65, 49, 30, 15 IR (below): Note that none of the choices has an O-H group, so the peak at 3500 must be for water impurity? LOD TRANSMETTANCEI
H,CO A в H. D Proton NMR: 7.1 (doublet, 2H), 6.9 (doublet, 2H), 4.0 (quartet, 2H), 3.5 (singlet, 2H), 2.2 (singlet, 3H), 1.3 (triplet, 3H) Carbon-13 NMR: 205, 157, 130, 126, 114, 65, 49, 30, 15 IR (below): Note that none of the choices has an O-H group, so the peak at 3500 must be for water impurity? LOD TRANSMETTANCEI
Chapter13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Section13.SE: Something Extra
Problem 66GP: A 13C NMR spectrum of commercially available 2,4-pentanediol, shows five peaks at 23.3, 23.9, 46.5,...
Related questions
Question
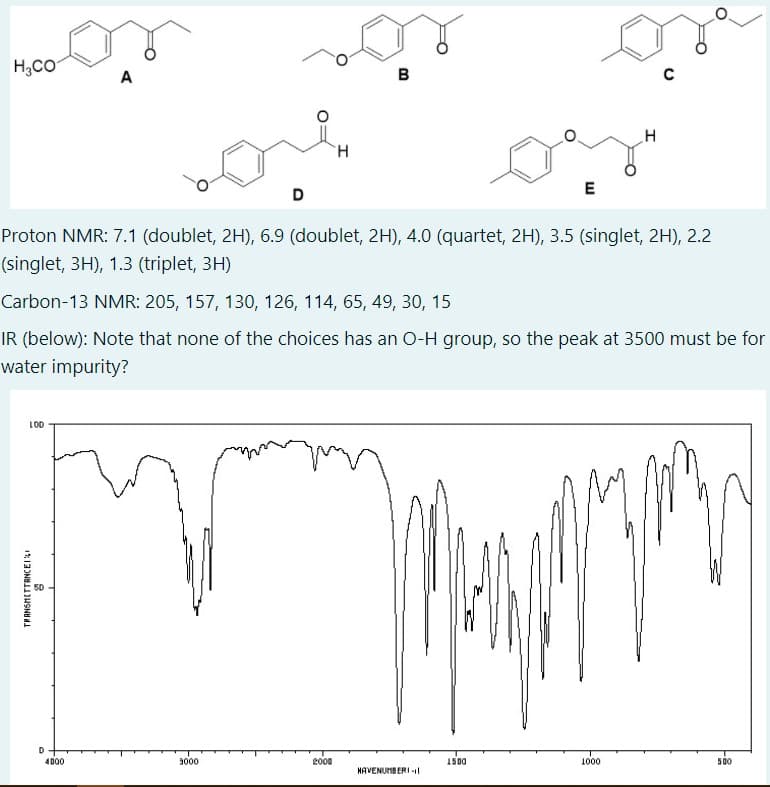
Transcribed Image Text:H,CO
A
B
C
H.
E
D
Proton NMR: 7.1 (doublet, 2H), 6.9 (doublet, 2H), 4.0 (quartet, 2H), 3.5 (singlet, 2H), 2.2
(singlet, 3H), 1.3 (triplet, 3H)
Carbon-13 NMR: 205, 157, 130, 126, 114, 65, 49, 30, 15
IR (below): Note that none of the choices has an O-H group, so the peak at 3500 must be for
water impurity?
LOD
D
4D00
3000
2000
1500
1000
50
NAVENUMBERI -1
TRANSHETTANCEI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

