he amide 4 has a nitrogen attached to the benzene ring, and a chlorine attached to a primary arbon. Yet, it doesn't react with itself in a nucleophilic displacement. Why is the nitrogen in the mide not nucleophilic? Give your answer in terms of the resonance forms of amides in general: ? R NH2
he amide 4 has a nitrogen attached to the benzene ring, and a chlorine attached to a primary arbon. Yet, it doesn't react with itself in a nucleophilic displacement. Why is the nitrogen in the mide not nucleophilic? Give your answer in terms of the resonance forms of amides in general: ? R NH2
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.68P: Show how the synthetic scheme developed in Problem 23.67 can be modified to synthesize this...
Related questions
Question
Could you help me with this question? I don't know where to start. All the information has been provided.
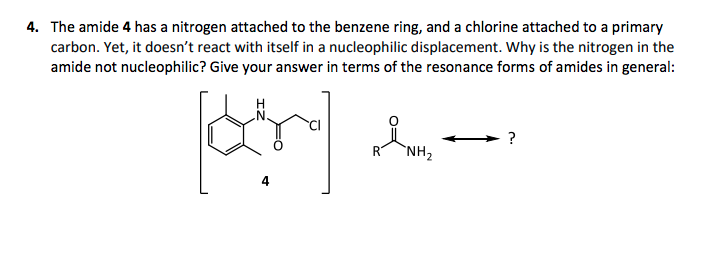
Transcribed Image Text:4. The amide 4 has a nitrogen attached to the benzene ring, and a chlorine attached to a primary
carbon. Yet, it doesn't react with itself in a nucleophilic displacement. Why is the nitrogen in the
amide not nucleophilic? Give your answer in terms of the resonance forms of amides in general:
?
RT
`NH2
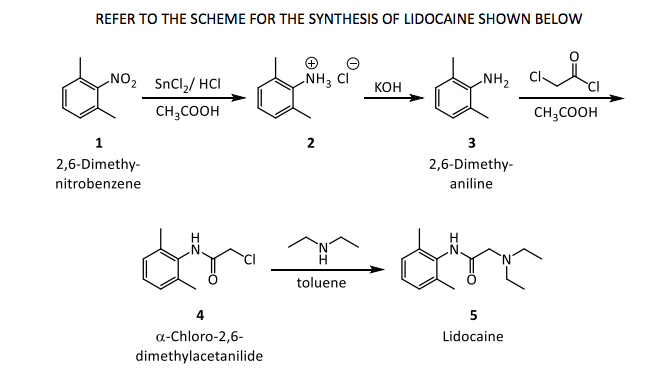
Transcribed Image Text:REFER TO THE SCHEME FOR THE SYNTHESIS OF LIDOCAINE SHOWN BELOW
NO2 SnCl,/ HCI
NH3 CI
NH2
Кон
CH,COOH
CH;COOH
1
2
3
2,6-Dimethy-
2,6-Dimethy-
nitrobenzene
aniline
H
toluene
5
a-Chloro-2,6-
Lidocaine
dimethylacetanilide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning