In certain clinical situations, there is need for an injectable B-blocker with a short biological half-life. The clue to development of such a drug was taken from the struc- ture of atenolol, whose corresponding carboxylic acid (the product of hydrolysis of its amide) has no B-blocking activity. Substituting an ester for the amide group and lengthening the carbon side chain by one methylene group resulted in esmolol. Its ester group is hydrolyzed quite rapidly to a carboxyl group by serum esterases under physiological conditions. This hydrolysis product has no B-blocking activity. OMe + CI HO NH2 4-Hydroxycinnamic acid Esmolol Isopropyl- amine Epichloro- hydrin
In certain clinical situations, there is need for an injectable B-blocker with a short biological half-life. The clue to development of such a drug was taken from the struc- ture of atenolol, whose corresponding carboxylic acid (the product of hydrolysis of its amide) has no B-blocking activity. Substituting an ester for the amide group and lengthening the carbon side chain by one methylene group resulted in esmolol. Its ester group is hydrolyzed quite rapidly to a carboxyl group by serum esterases under physiological conditions. This hydrolysis product has no B-blocking activity. OMe + CI HO NH2 4-Hydroxycinnamic acid Esmolol Isopropyl- amine Epichloro- hydrin
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.60P
Related questions
Question
Propose a synthesis for esmolol from 4-hydroxycinnamic acid, epichlorohydrin, and isopropylamine
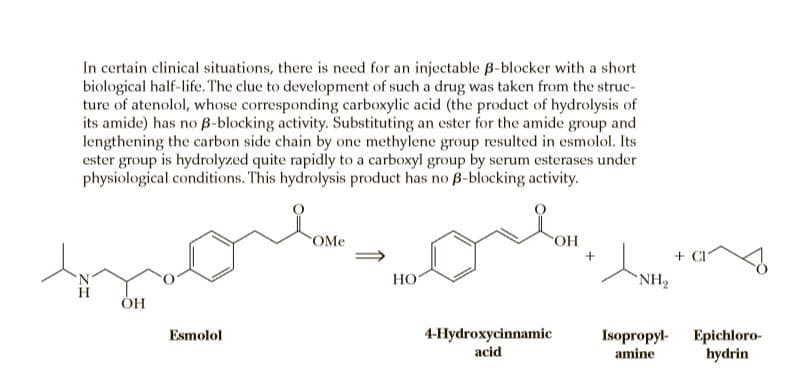
Transcribed Image Text:In certain clinical situations, there is need for an injectable B-blocker with a short
biological half-life. The clue to development of such a drug was taken from the struc-
ture of atenolol, whose corresponding carboxylic acid (the product of hydrolysis of
its amide) has no B-blocking activity. Substituting an ester for the amide group and
lengthening the carbon side chain by one methylene group resulted in esmolol. Its
ester group is hydrolyzed quite rapidly to a carboxyl group by serum esterases under
physiological conditions. This hydrolysis product has no B-blocking activity.
OMe
+ CI
HO
NH2
4-Hydroxycinnamic
acid
Esmolol
Isopropyl-
amine
Epichloro-
hydrin
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
