he following alkene undergoes hydroboration-oxidation to yield a single product rather than a mixture. Explain the result, and draw the product show- ing its stereochemistry.
he following alkene undergoes hydroboration-oxidation to yield a single product rather than a mixture. Explain the result, and draw the product show- ing its stereochemistry.
Chapter14: Conjugated Compounds And Ultraviolet Spectroscopy
Section14.SE: Something Extra
Problem 50AP: -Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the...
Related questions
Question
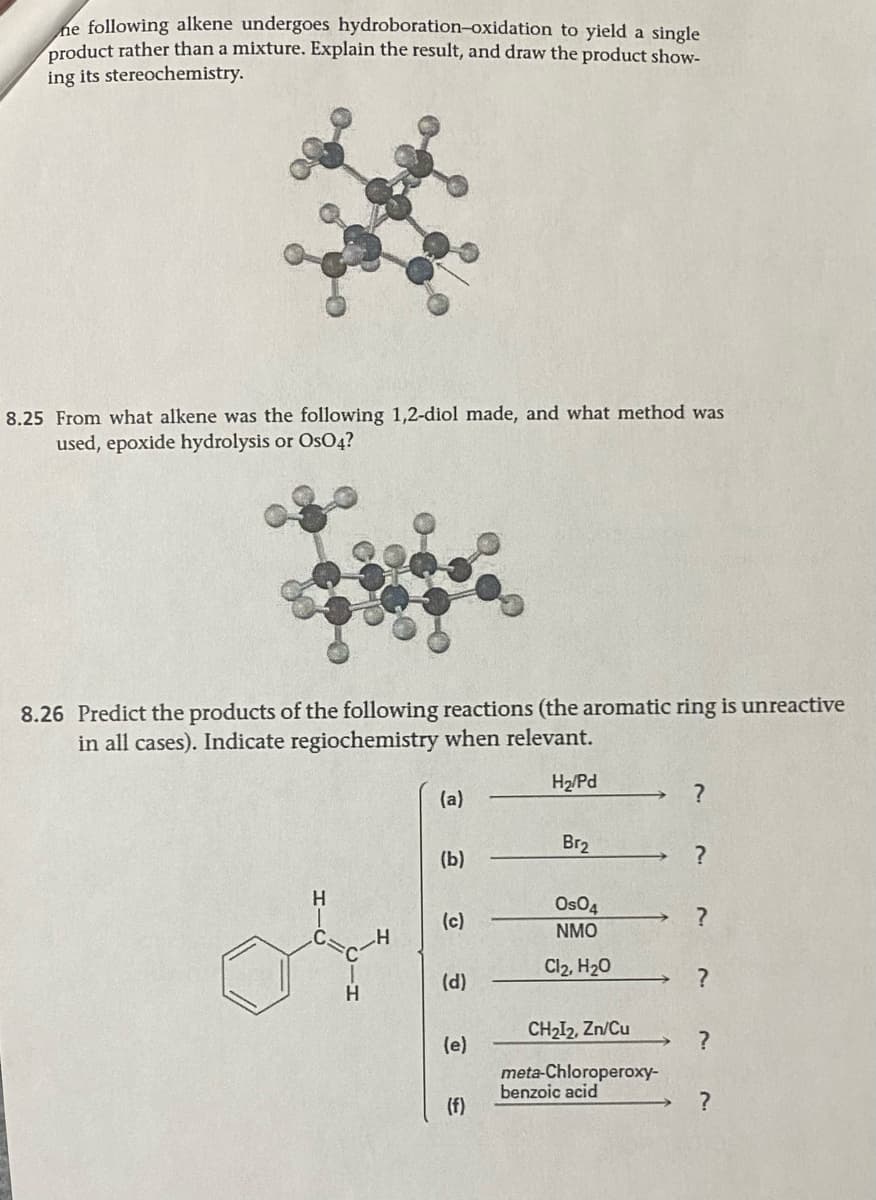
Transcribed Image Text:he following alkene undergoes hydroboration-oxidation to yield a single
product rather than a mixture. Explain the result, and draw the product show-
ing its stereochemistry.
8.25 From what alkene was the following 1,2-diol made, and what method was
used, epoxide hydrolysis or OsO4?
8.26 Predict the products of the following reactions (the aromatic ring is unreactive
in all cases). Indicate regiochemistry when relevant.
H₂/Pd
H
or
(a)
(b)
(c)
(d)
(e)
(f)
Br₂
Os04
NMO
Cl₂, H₂O
CH₂I₂, Zn/Cu
meta-Chloroperoxy-
benzoic acid
?
?
?
?
?
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning