Q: A closed, 7.8-L flask contains 1.0 g of water. At what temperature will half of the water be in t…
A: Solution -
Q: Trypsin is a serine protease that has a KM of 15 mM. At an initial substrate concentration of 250 µM…
A: Given that, for a serine protease trypsin The Michaelis-Menten constant is KM = 15 mM = 15×10-3 M,…
Q: 4. Which of the foillowing examples demonstrates Charles' Law? A. hot air balloon B. lungs 5. If…
A:
Q: It was discovered that a beverage stored at room temperature (assume 25 degrees C) spoils 40 times…
A: The relationship between rate constant , temperature and activation energy is given by Arrhenius…
Q: *19. Following are the NMR spectra of three isomeric esters with the formula C;H14O2, all derived…
A: First we have to calculate the double bond equivalence (DBE) of the moleculer formula C7H14O2 by…
Q: 15.) What is the pH at the equivalence point in the titration of 100.0 mL of 0.10 M HCI with 0.10 M…
A:
Q: 1. CH,OH Br Br2 HBr ÓCH, 2. CH2 Li a = Proton transfer d = Radical chain addition g = E2 Elimination…
A: The mechanism by which the reaction proceeds is:
Q: The orbital diagram of 13AI has , unpaired electron(s). O 1 O 2 O 3 О 13
A: Aufbau's principle :- It states that electrons are filled into the orbitals in increasing order of…
Q: QUESTION 7 a) Sketch all the steps required for the following synthesis. но. Compound H b) Write the…
A:
Q: 1.876 g of the sample containing H2C2O4requires 38.84 mL of 0.1032 M of NaOH for titration: H2C2O4+…
A: Given that - Mass of sample containing H2C2O4 = 1.876 g Volume of NaOH = 38.84 mL Molarity of…
Q: At 25 °C, the following reactions have the equilibrium constants noted to the right of their…
A: Equilibrium constant When coefficient are changed: If a balanced chemical equation coefficient are…
Q: For the following 9 compounds (most of which were the ones used in this lab), tell me what peaks you…
A: A question based on NMR spectroscopy that is to be accomplished.
Q: Which of the following describe(s) the following reaction? Choose all that apply. 235TJ 92 1Xe +…
A: Chain reaction ===> Process in which neutrons released in fission produce an additional fission…
Q: H H. В エ Z-エ エ の A
A: ->Conjugate acid of bases have same order of Basicity.(or) conjugate acid of strongest base is…
Q: а) H TO H,B-H b) HN
A:
Q: Differentiate the scents of Lemon and Orange extracts
A: Lemon and orange extract are the flavouring material use to add flavour along with that these are…
Q: For each of the following, provide the product or reagent as necessary H20 NaBH4 + Hg(OAc)2 ELOH а)…
A:
Q: What is the importance of having a titration of about pH 10 in compleximetric titration?
A: In complexometric titration end point is observed by the formation coloured complex.
Q: Which of the following characterizes a gamma ray? Choose all that apply. |is more penetrating than a…
A: Energy of gamma rays are more in energy than X-rays according to EMR spectra series though both…
Q: (a) Delineate the outcome of the foliowing reactions with mechanism : -NO2 diexan (i) reflux Ph…
A:
Q: Which of the following characterizes an alpha ray? Choose all that apply. O is attracted to the…
A: A) is attracted to the negatively charged plate in an electric field.
Q: Below is the structure of a hypothetical photo-affinity probe analogue of the anti- malarial drug…
A: Cross-links with nearby proteins in the presence of UV light. B is the correct answer.
Q: solve question 3, Q3: Calculate the freezing point of an antifiveze (100g) sebation that is 50 0%…
A: Given that - Mass of ethylene glycol containing Solution = 100 g Then, mass of ethylene glycol ,…
Q: How would you prepare the following compounds, starting with cyclopentene and any other reagents…
A: Markovnikov’s Rule When a protic acid (HX) is added to an asymmetric alkene, the acidic hydrogen…
Q: Cl-C-C-Br ČI HB НА НВ *10. Explain the patterns and intensities of the isopropyl group in isopropyl…
A: The correct answer about NMR spectrum is given below
Q: How many moles are there in 176 g of Octane (C3H18)? O 0.57 O 1.54 O 0.65 O 1.76
A: Given, compound = Octane mass of the given octane = 176 g Molecular formula = C8H18…
Q: 1. Solid barium nitrate is slowly added to 75.0 mL of a 0.0478 M ammonium sulfite solution. The…
A:
Q: ce (OH ].
A:
Q: Cd(s)Cd2*(aq)||Cu2+(aq)|Cu(s) O Cd (s) > Ca2* (aq) + 2e O Cu?* (aq) + 2e Cu (s)
A:
Q: Specific Chemical Shift multiplicity (s, d, t, etc.) Integration # of 13C-NMR Br Proton (ppm) (# of…
A: Chemical shift- It is the resonant frequency of a nucleus relative to a standard in a magnetic…
Q: In both examples below the reactants shown are combined to bring about a nucleophilic substitution…
A:
Q: Match the region A,B,C. Question 4 Below is the structure of a hypothetical photo-affinity probe…
A: 1. Cross-links with nearby proteins in the presence of UV light The answers is B.
Q: 11. Given the following thermochemical data: 1. C,H;(g) +½ 0:(g) → 2CO;(g) + H;O(1) 2. C,H(g) + 2 O;…
A: If one reaction is the combination of more than one reaction then the enthalpy of the reaction is…
Q: Which of the following do NOT show the presence of benzylic hydrogen? 1,2-diiodobenzene…
A: The carbon atom attached to the benzene ring is known as benzylic carbon and the hydrogen atom…
Q: A student is trying to prepare a buffer solution with a pH of 9.00. Calculate the mass of potassium…
A: pH of a buffer is calculated using Henderson -Hasselbalch equation pH = 9 pka = -log Ka We…
Q: Which of the scenarios below can happen if hot coffee is poured into cold water? Explain. The…
A: According to the second law of thermodynamics the heat energy can flow spontaneously from a higher…
Q: A student bubbled sulphur dioxide gas into acidified potassium permanganate solution. a.Write an…
A: Given, A student bubbled sulphur dioxide gas into acidified potassium permanganate solution. a.…
Q: Using the cell potentials found on page 815 of your textbook, calculate the equilibrium constant for…
A: Sr(s) + Ca2+ ---> Sr2+ + Ca(s)
Q: 3) Patient with CPS1 deficiency, what will happen to theses processes ? (write : increase, decrease,…
A: Solution- Patient with CPS1 deficiency- CPSID (carbamoyl phosphate synthetase 1 deficiency) is a…
Q: A Sodium Chloride (0.1920g) NaCl was assayed using volhard Method, using 52mL of 0.0975N AGNO3 and…
A: Given that - Mass of NaCl = 0.1920 g Normality of AgNO3 = 0.0975 N Volume of AgNO3 = 52 mL…
Q: 19-1 Review A harmonic oscillator is vibrating at a frequency of 6.11x1014s. Use the harmonic…
A:
Q: Consider the titration of 50.0 mL of 0.0500 M NaCl with 0.100 M AgNO3. Calculate pCl at various…
A: 1,2
Q: In the analysis of a sample for KH2PO4content, a sample weighing 0.3994 g required 18.28 mL of…
A:
Q: At room temperature, how would you expect the Kof AgCl in deionized water to compare to the K.of…
A: Answer: Solubility equilibrium AgCl is shown below: AgCl(s)↔Ag+(aq)+Cl-(aq)
Q: Arrange in order of decreasing acidi
A: A substance is said to be acidic if can donate or lose H+ ion The strength of acid depends on the…
Q: 2RO, (aq) + 12 H* (aq) + 3 M (s) = RO (g)+3M²* (aq) + 6 H20 (1) = 3.86 V. R: 5 valence e-,…
A: In the given reaction RO3- reacts with M to form RO And M2+
Q: Why are indicators useful?
A: To explain: Uses of the indicator.
Q: Find the boiling point (Tb) of a solution containing 15.0 g salt (NaCl) dissolved in 750 g water
A: We're asked to find the expected boiling point elevation of a solution given the amount of solute…
Q: Identify 2 cosmetic ingredients (either in cosmetic or toiletry products) that are classified under…
A: We have to find 2 cosmetic ingredients comes under the categories of Skin sensitizer. Skin irritant,…
Q: Clearly indicate the acid and the conjugate acid in each of the following reactions and determine…
A: Equilibrium is favoured on the side where more stable product is formed. Positive charge is more…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- By the use of Henderson Hasselbalch equation; pH = pKa + log{[acetate ion]/[acetic acid]} 4.5 = 4.75 + log{[0.10 M]/[acetic acid]} -0.25 = log{[0.10 M]/[acetic acid]} [Acetic acid] = 0.10 M/ 10-0.25 [Acetic acid] = 0.10 M/0.56 [Acetic acid] = 0.1786 M Moles of sodium acetate dissolved in 250 mL buffer solution = 0.10 M× (250mL/1000mL) × 1L = 0.025 mol Weight (w) of sodium acetate (purity 100%) dissolved to prepare 250 mL of solution with buffer concentration of 0.10 M is calculate as follow; w100% = 0.025 mol × 82.0343 g/mol = 2.051 g Weight (w) of sodium acetate (purity 99%) is calculate as follow; w99% = 2.051 g× (100/99) = 2.072 g What was the volume of 6.12 M acetic acid HC2H3O2 needed to prepare the 250 mL acetic acid/acetate ion buffer solution required in this part? Show your calculations.The following evidence was obtained from an experiment to determine the solubility of calcium chloride at room temperature. A sample of saturated calcium chloride solution was evaporated to dryness, and the mass of solid residue was measured.EvidenceVolume of solution (mL) = 15.0Mass of empty beaker (g) = 90.54Mass of beaker and residue (g) = 101.36The solubility of calcium chloride is g/100 mLFor an aqueous solution saturated in both AgCl and AgI, at 1 bar and at 298 K. The equilibrium constants for this system are given as follow: Ksp(AgCl) = 1.8*10^-10, Ksp(AgI) = 8.5*10^-17, and Kw = 1.0*10^-14. How many formalities must be given to get unique values for all the concentrations?
- Chloroform (1)/methyl ethyl ketone (2) form a solution in vapor liquid equilibrium at 330 K. The chloroform concentration in the liquid phase is x1 = 0.55. Assuming an ideal gas and an ideal solutionof liquids, what are y1 and P (in kPa), to 3 significant figures?Hexanoic acid was added to an immiscible biphasic solvent sysem, water and CCl4 at 20.0OC and the equilibrium concentrations of hexanoic acid were determined to be 3.66 g/L in H2O and 67.0 g/L in CCl4. Caluclate the distrubution coeffiecent (D1) of hexanoic acid in CCl4 with respect to water.A 1.00-m solution of acetic acid, CH3COOH, in benzene has a freezing point of 2.96°C. Use the data in the Table to calculate the value of i and suggest an explanation for the unusual result. (Hint: If i is less than 1.0, each formula unit that dissolves yields less than one solute particle, an outcome suggesting aggregation of solute particles.) Answer is: i = 0.50; formation of dimers of composition (CH3COOH)2, need steps shown to understand though
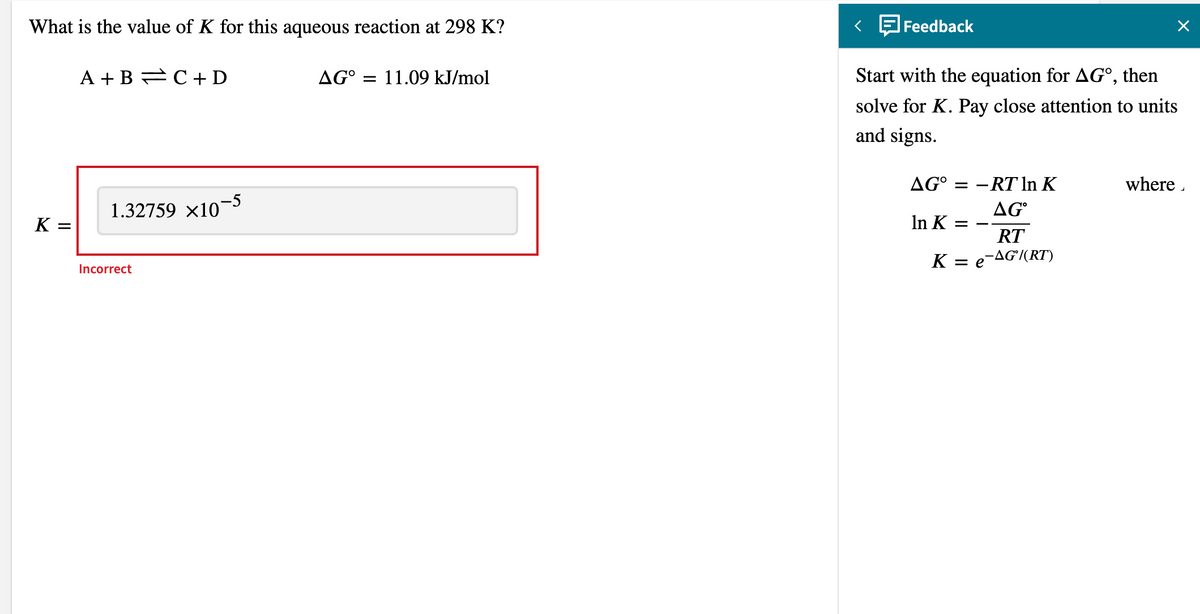
- Consider an aqueous solution saturated in both AgCl and AgI, at 1 bar and at 298 K. The equilibrium constants for this system are Ksp(AgCl) = 1.8*10-10E-10, Ksp(AgI) = 8.5*10E-17 and Kw = 1.0*10E-14. Find the concentration of all the ionic species. If you need to specify any formalities, make them 1 M.Hexanoic acid was added to an immiscible biphasic solvent system, water and CCl4 at 20.0OC and the equilibrium concentrations of hexanoic acid were determined to be 3.66 g/L in H2O and 67.0 g/L in CCl4. Caluclate the distrubution coeffiecent (D2) of hexanoic acid in water with respect to CCl4.What is the value of the equilibrium constant when ΔGorxn = 25 kJ·mol-1 and T=298 K?
- You have a calorimeter with a heat capacity of 0.426 kJ/ºC. You combine 50.0 mL 1.50 M NaA (A- = conjugate base of weak acid HA) with an equal volume of HCl of higher molarilty. The temperature changes from 18.6 ºC to 21.1 ºC. What is ΔHºdissoc for the dissociation reaction of HA, in kJ?Calculate the solubility at 25°C of PbCO3 in pure water and in a 0.0110M PbNO32 solution. You'll find Ksp data in the ALEKS Data tab. Round both of your answers to 2 significant digits. solubility in pure water: gL solubility in 0.0110 M Pb(NO3)2solution: gLIn the synthesis of hydrocarbons, the carbon source is carbon dioxide. Although the CO2 concentra?on in the atmosphere raises at a drama?c speed, point sources are probably the easier sources for a PtX process. Iden?fy 3 possible point sources, explain why CO2 is formed and what challenges each of the three CO2 streams presents

