Hello I need help with the following 3 questions. I need the following questions done before I can do the next part of my experiment. Here is the information needed to solve them. 1. Create a graph of the titration curve of 20 mL sudsy ammonia with 0.5 M citric acid. Be sure to title your graph and label your axes. 2. What is the pH of the solution at the equivalence point shown by the titration curve? 3. Which indicator shows a color change at about the same pH as the equivalence point?
Hello I need help with the following 3 questions. I need the following questions done before I can do the next part of my experiment. Here is the information needed to solve them. 1. Create a graph of the titration curve of 20 mL sudsy ammonia with 0.5 M citric acid. Be sure to title your graph and label your axes. 2. What is the pH of the solution at the equivalence point shown by the titration curve? 3. Which indicator shows a color change at about the same pH as the equivalence point?
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.9QAP
Related questions
Question
Hello I need help with the following 3 questions. I need the following questions done before I can do the next part of my experiment. Here is the information needed to solve them.
1. Create a graph of the titration curve of 20 mL sudsy ammonia with 0.5 M citric acid. Be sure to title your graph and label your axes.
2. What is the pH of the solution at the equivalence point shown by the titration curve?
3. Which indicator shows a color change at about the same pH as the equivalence point?
Transcribed Image Text:AutoSave
GC 4253_L20_..
Search
Cabrera, Aleson (acabrera14)
Of
CA
File
Home
Insert
Draw
Design
Layout
References
Mailings
Review
View
Help
A Share
O Commer
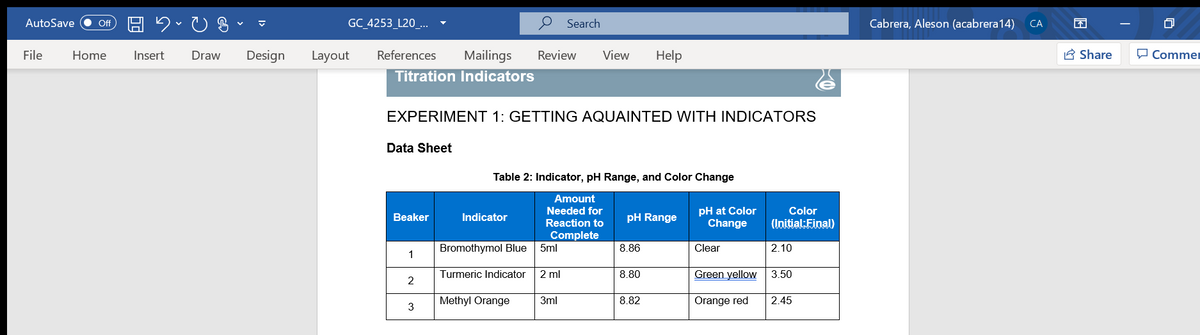
Titration Indicators
EXPERIMENT 1: GETTING AQUAINTED WITH INDICATORS
Data Sheet
Table 2: Indicator, pH Range, and Color Change
Amount
pH at Color
Change
Needed for
Color
Beaker
Indicator
pH Range
Reaction to
(Initial:Final)
Complete
Bromothymol Blue
5ml
8.86
Clear
2.10
1
Turmeric Indicator
2 ml
8.80
Green yellow
3.50
2
Methyl Orange
3ml
8.82
Orange red
2.45
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning