help with study guide, thank you!
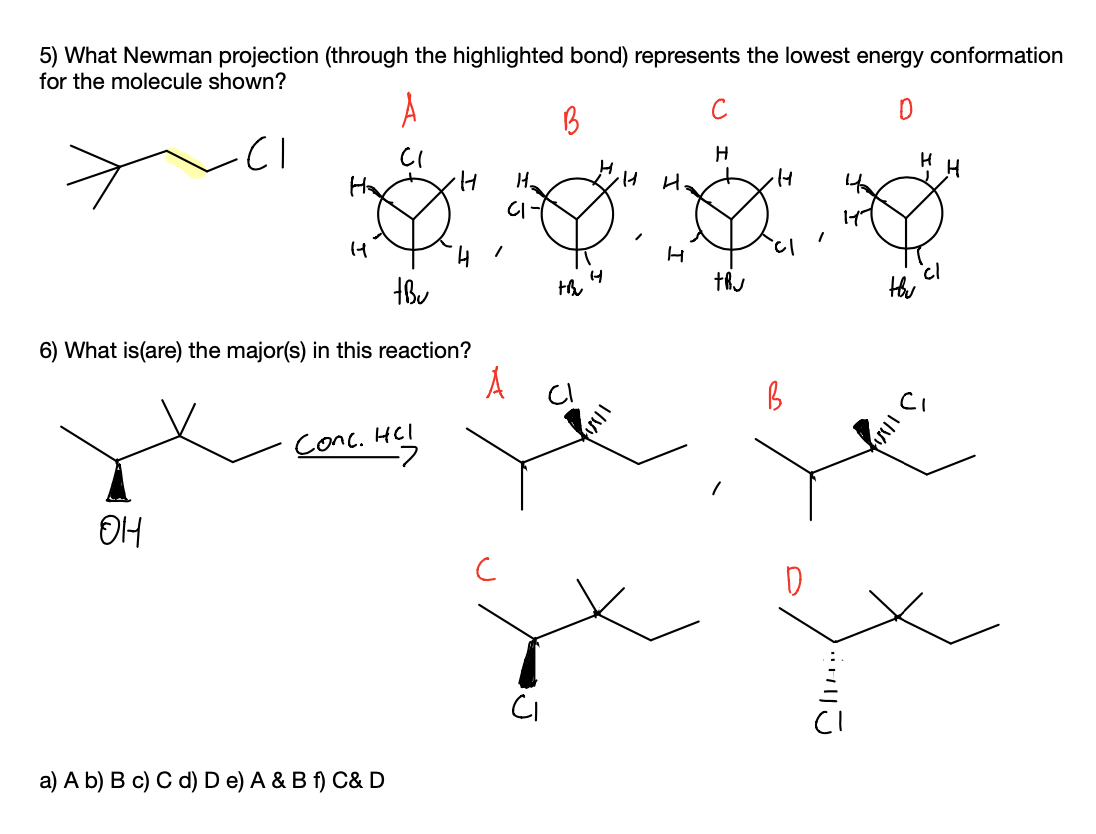
5)Newmann projection- we have to visualize the structure of the compound from front to back carbon bonds.
eclipsed conformation of Newmann projection the groups are more closer, create more torsional strain.
staggered conformation of Newmann projection the groups are far away from each other, create less torsional strain.
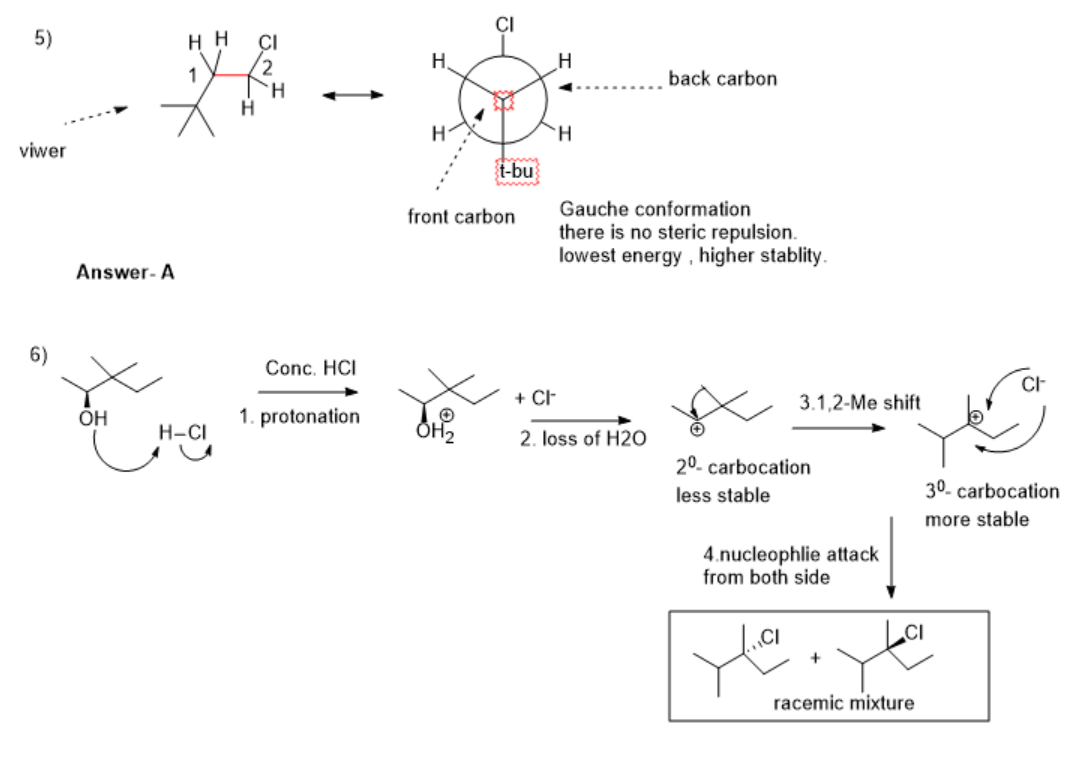
Answer-In conformation A- there is no torsional strain between the H and Cl group, and the H and t-butyl group, due to this there is less energy and higher stability.
6) step 1. protonation of alcohol by Strong acid HCl.
step 2. loss of leaving group H2O, forming 20-carbocation( less stable)
Step 3.Rearrangement- 1,2-Me shift occurs for the formation of 30-carbocation( more stable).
Step 4. a nucleophilic attack by Cl- occurs from both sides, the formation of the racemic mixture as a product.
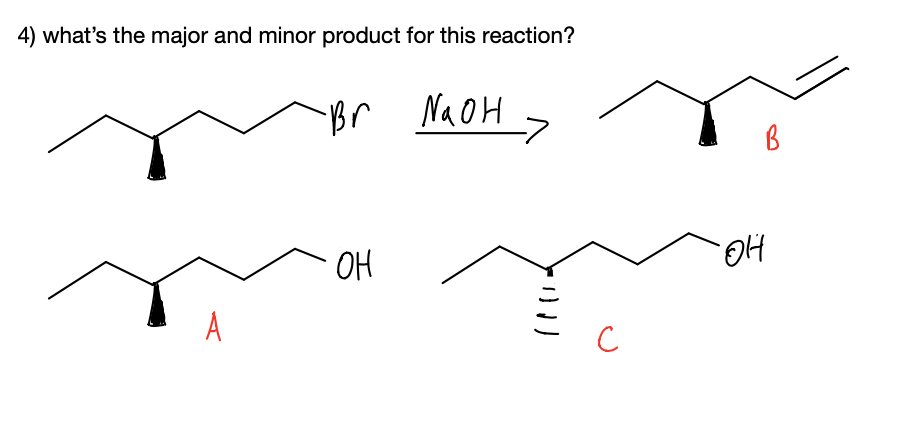
Answer- e) A & B
Step by step
Solved in 2 steps with 2 images









