here was a hd, new temperature hat woud be graphed in green and would display the effect of a greater, even higher temperature than HigherT on the distibution of netic energes of molecules nasample, descrbe the shape and relative height of this ne green ouve astreates to the exsting red gher Toune in the graph Lower T Minimum energy needed for reaction, E, Higher T Drag the words on the let to the aporopriate blaks on the nght to complete the sentence Larger fraction of molecules reacts at higher temperature let .For a higher temperatue, he urve would be lower out, the maemum of the cunve would be of the maximum of the red curve, and higher Kinetic energy Faction of moecuies would have ineic emegy grea right The graph shown displays the effect of two different temperatures on the distribution of kinetic energies of molecules in a sample. One temperature is named "LowerT and is graphed in blue. The other temperature is named "Higher Tand is graphed in red. the red ounve more less greater smaler Reset Meip 1. For a higher temperature, the curve would be spread out, the maximum of the curve would be and to the of the maximum of the red curve, and a fraction of molecules would have kinetic energy greater than E, than for the red curve. Fraction of molecules
here was a hd, new temperature hat woud be graphed in green and would display the effect of a greater, even higher temperature than HigherT on the distibution of netic energes of molecules nasample, descrbe the shape and relative height of this ne green ouve astreates to the exsting red gher Toune in the graph Lower T Minimum energy needed for reaction, E, Higher T Drag the words on the let to the aporopriate blaks on the nght to complete the sentence Larger fraction of molecules reacts at higher temperature let .For a higher temperatue, he urve would be lower out, the maemum of the cunve would be of the maximum of the red curve, and higher Kinetic energy Faction of moecuies would have ineic emegy grea right The graph shown displays the effect of two different temperatures on the distribution of kinetic energies of molecules in a sample. One temperature is named "LowerT and is graphed in blue. The other temperature is named "Higher Tand is graphed in red. the red ounve more less greater smaler Reset Meip 1. For a higher temperature, the curve would be spread out, the maximum of the curve would be and to the of the maximum of the red curve, and a fraction of molecules would have kinetic energy greater than E, than for the red curve. Fraction of molecules
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter18: Chemical Equilibrium
Section: Chapter Questions
Problem 83E
Related questions
Question
Solve it completely otherwise I will downvote
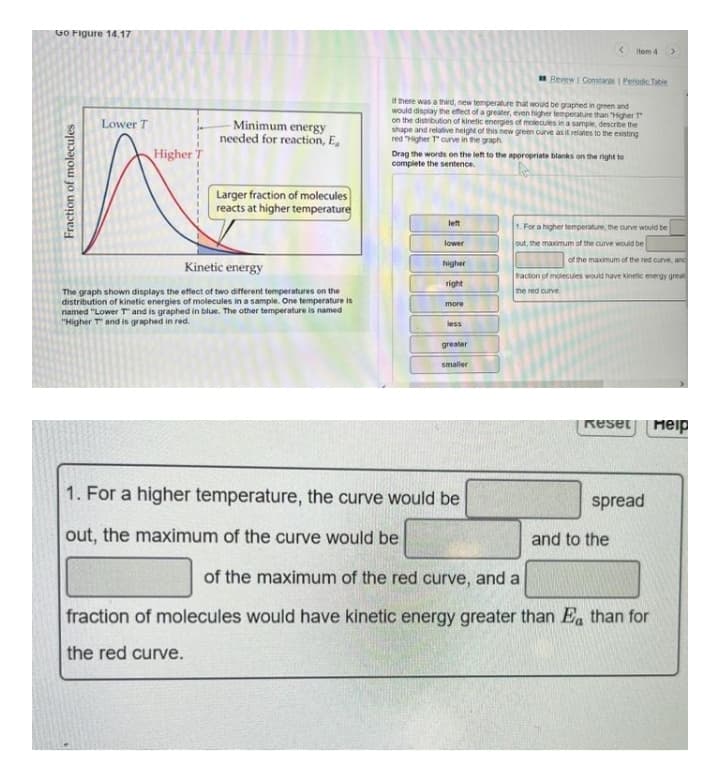
Transcribed Image Text:Go Figure 14.17
Itom 4
Revew I Constants I Perodc Tabie
It there was a thid, new temperature that would be graphed in green and
would display the efect of a greater, even higher temperature than HigherT
on the distribution of kinetic energies of molecules in a sample, describe the
shape and relative height of this new green curve as it relates to the existing
red Higher Tcurve in the graph
Lower T
Minimum energy
needed for reaction, E,
Higher T
Drag the words on the left to the appropriate blanks on the right to
complete the sentence.
Larger fraction of molecules
reacts at higher temperature
let
1. For a higher temperature, me aurve would be
lower
out, the maximum of the curve would be
Kinetic energy
higher
of the maximum of the red curve, anc
traction of moecuies would have kinetic energy great
right
me red curve
The graph shown displays the effect of two different temperatures on the
distribution of kinetic energies of molecules ina sample. One temperature is
named "Lower T and is graphed in blue. The other temperature is named
"Higher T and is graphed in red.
more
less
greater
smaller
Reset
Meip
1. For a higher temperature, the curve would be
spread
out, the maximum of the curve would be
and to the
of the maximum of the red curve, and a
fraction of molecules would have kinetic energy greater than Ea than for
the red curve.
Fraction of molecules
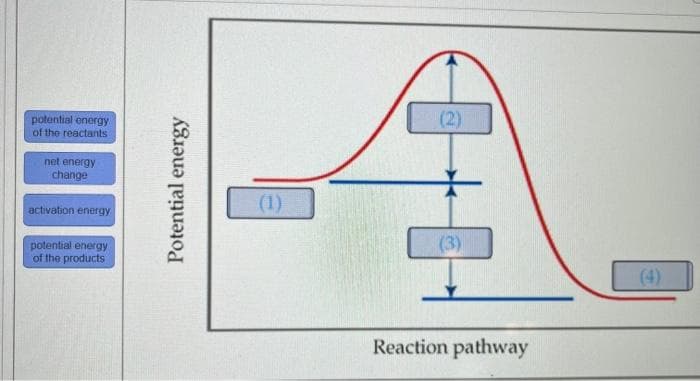
Transcribed Image Text:potential onergy
of the reactants
(2)
net energy
change
(1)
activation energy
potential energy
of the products
(3)
(4)
Reaction pathway
Potential energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
