Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.SE: Something Extra
Problem 52AP: Using molecular models as well as structural drawings, explain why trans-decalin is rigid and cannot...
Related questions
Question
Quinapril (trade name Accupril) is used to treat high blood pressure and congestive heart failure. One step in the synthesis of quinapril involves reaction of the racemic alkyl bromide A with a single enantiomer of the amino ester B. (a) What two products are formed in this reaction? (b) Given the structure of quinapril, which one of these two products is needed to synthesize the drug?
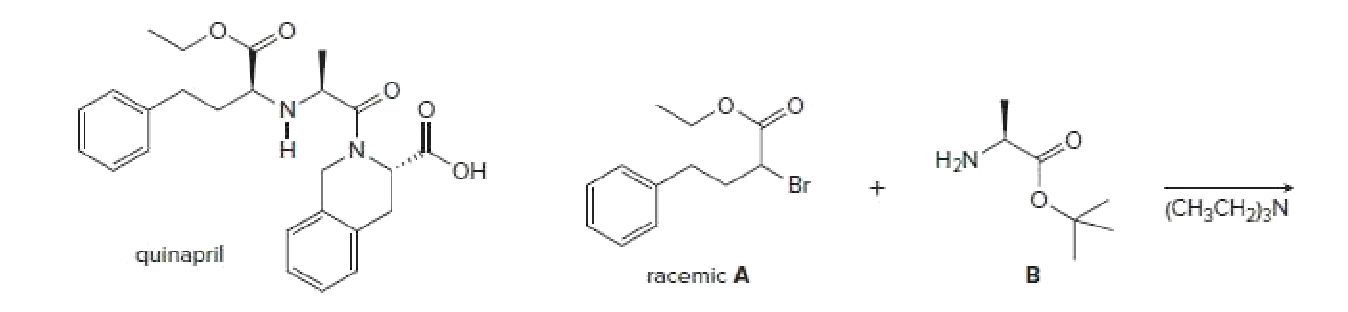
Transcribed Image Text:H;N
HO.
Br
(CH3CH2}3N
quinapril
racemic A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning