HO, oxybenzone se Beer's Law and data provided in the table to determine how many milligrams of oxybenzone are present in 2.5 mL of nscreen. olar mass of oxybenzone = 228.2 g/mol Molar Extinction Pathlength of UV Volume of Sunscreen Absorbance Wavelength Coefficient cell Sample 0.95 290 nm 14365 M-'cm-l 1.0 cm 2.5 mL Iow many milligrams of oxybenzone are present in 2.5 mL of sunscreen? mg
HO, oxybenzone se Beer's Law and data provided in the table to determine how many milligrams of oxybenzone are present in 2.5 mL of nscreen. olar mass of oxybenzone = 228.2 g/mol Molar Extinction Pathlength of UV Volume of Sunscreen Absorbance Wavelength Coefficient cell Sample 0.95 290 nm 14365 M-'cm-l 1.0 cm 2.5 mL Iow many milligrams of oxybenzone are present in 2.5 mL of sunscreen? mg
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 6P
Related questions
Question
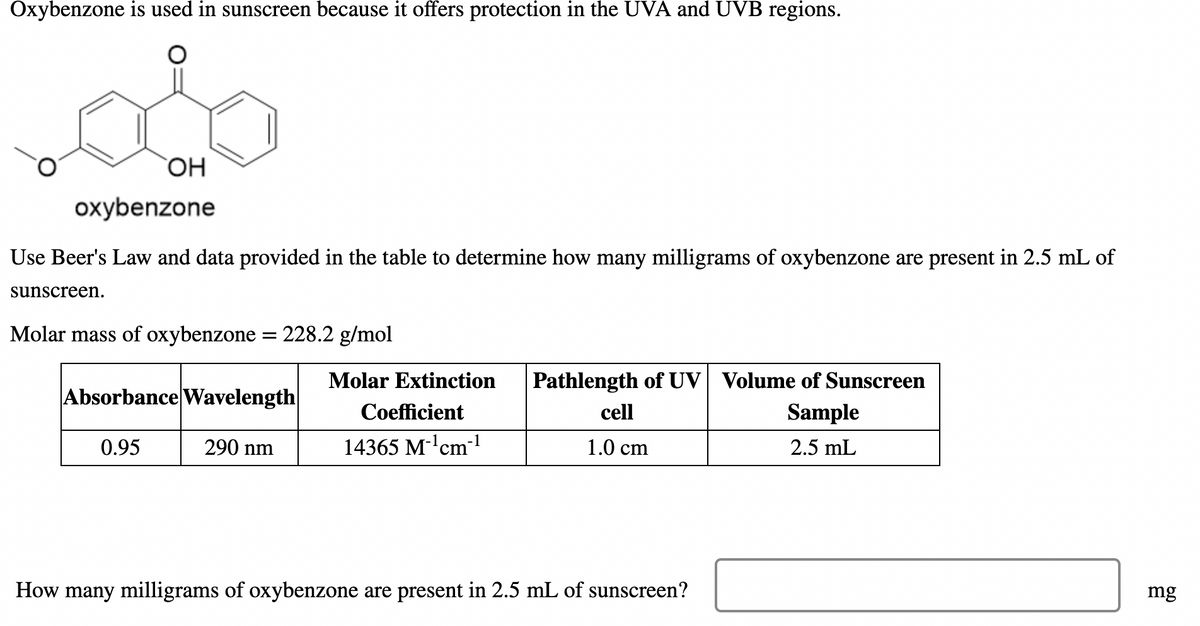
Transcribed Image Text:Oxybenzone is used in sunscreen because it offers protection in the UVA and UVB regions.
oxybenzone
Use Beer's Law and data provided in the table to determine how many milligrams of oxybenzone are present in 2.5 mL of
sunscreen.
Molar mass of oxybenzone = 228.2 g/mol
Molar Extinction
Pathlength of UV Volume of Sunscreen
Absorbance Wavelength
Coefficient
cell
Sample
0.95
290 nm
14365 M-'cm-1
1.0 cm
2.5 mL
How many milligrams of oxybenzone are present in 2.5 mL of sunscreen?
mg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning