Home X CHE101_02: Intro to General Che x 101 Chem 101 х app.101edu.co myClackamas Login CUnofficial Transcri... W Logon Oregon Scholarsh... Home - FAFSA on... Welcome to the O... The National Soci... Apps Submit Question 3 of 8 Determine the number of grams of CAH10 that are required to completely react to produce 8.70 mol of CO2 according to the following combustion reaction: 13 O2(g) H2O(g) C4H10(g) CO2(g) 2 8 10 X STARTING AMOUNT ADD FACTOR RESET ANSWER () X 18.02 208.00 4.35 126 13 0.0374 44.01 10 8.70 2.18 8 1 6.022 x 1023 2 58.14 7:19 PM e e Type here to search ENG 11/13/2019
Home X CHE101_02: Intro to General Che x 101 Chem 101 х app.101edu.co myClackamas Login CUnofficial Transcri... W Logon Oregon Scholarsh... Home - FAFSA on... Welcome to the O... The National Soci... Apps Submit Question 3 of 8 Determine the number of grams of CAH10 that are required to completely react to produce 8.70 mol of CO2 according to the following combustion reaction: 13 O2(g) H2O(g) C4H10(g) CO2(g) 2 8 10 X STARTING AMOUNT ADD FACTOR RESET ANSWER () X 18.02 208.00 4.35 126 13 0.0374 44.01 10 8.70 2.18 8 1 6.022 x 1023 2 58.14 7:19 PM e e Type here to search ENG 11/13/2019
Chapter6: Random Errors In Chemical Analysis
Section: Chapter Questions
Problem 6.16QAP
Related questions
Question
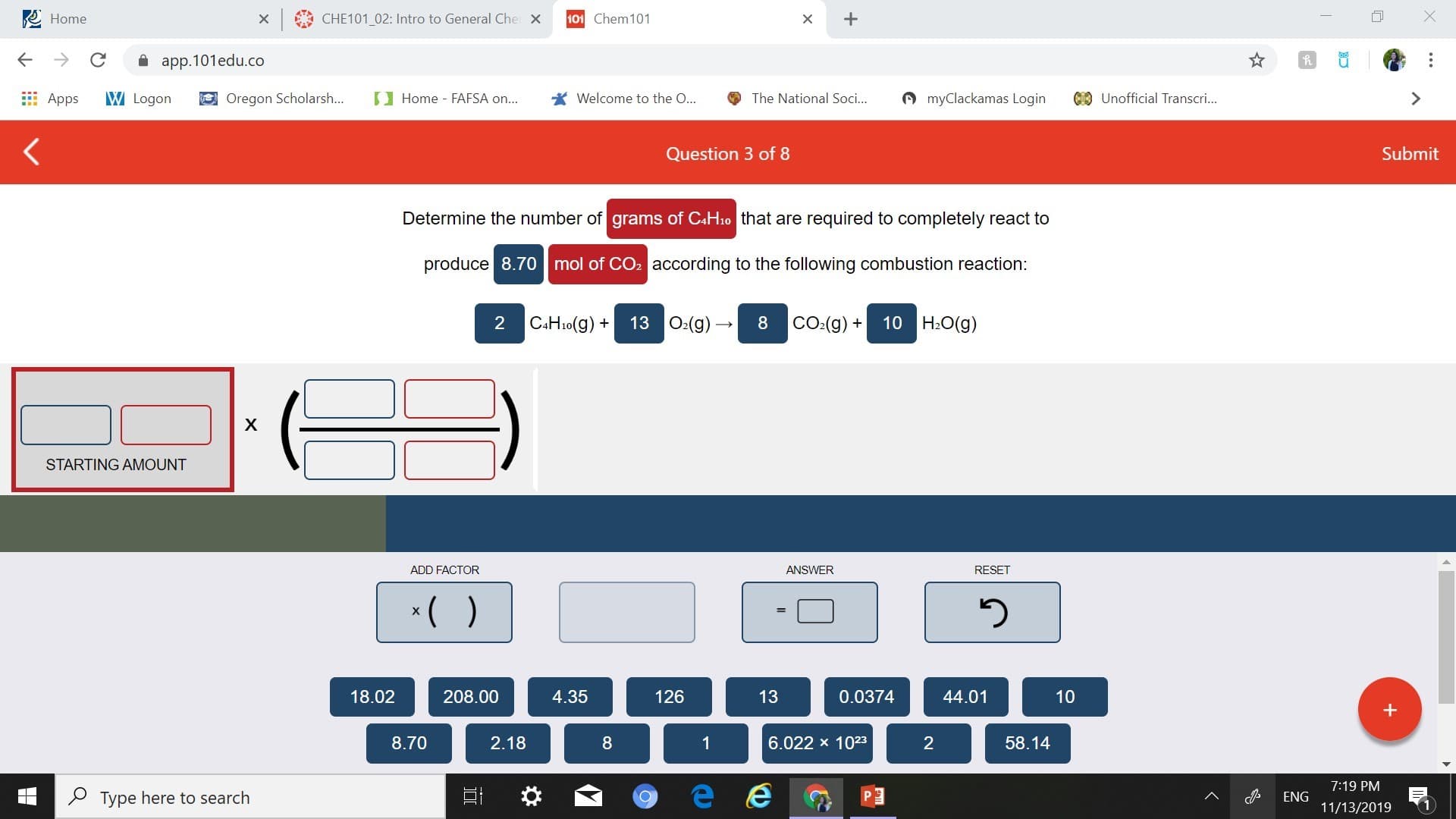
Transcribed Image Text:Home
X CHE101_02: Intro to General Che x
101 Chem 101
х
app.101edu.co
myClackamas Login
CUnofficial Transcri...
W Logon
Oregon Scholarsh...
Home - FAFSA on...
Welcome to the O...
The National Soci...
Apps
Submit
Question 3 of 8
Determine the number of grams of CAH10 that are required to completely react to
produce 8.70 mol of CO2 according to the following combustion reaction:
13 O2(g)
H2O(g)
C4H10(g)
CO2(g)
2
8
10
X
STARTING AMOUNT
ADD FACTOR
RESET
ANSWER
()
X
18.02
208.00
4.35
126
13
0.0374
44.01
10
8.70
2.18
8
1
6.022 x 1023
2
58.14
7:19 PM
e e
Type here to search
ENG
11/13/2019
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning