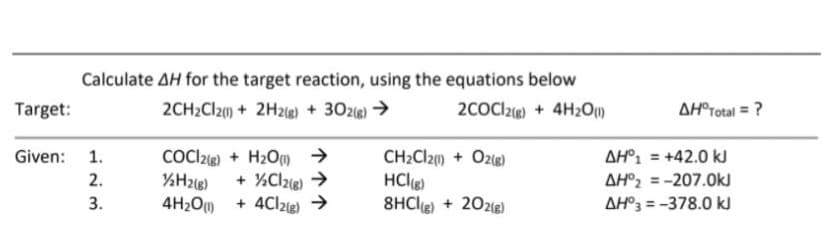
Calculate AH for the target reaction, using the equations below 2CH2CI2() + 2H2g) + 302e) > Target: 2COCl2ig) + 4H2O1) AH®rotal = ? Given: 1. COC22®) + H2Om > CH2CI20) + O2g) AH°1 = +42.0 kJ %3D %H2i8) + %Clze) > HClg) 8HCle) + 202le) 2. AH°2 = -207.0kJ %3D 4H2O + 4Clzg) → AH°3 = -378.0 ki 3.
Q: The below reaction was found to have AH = -406 kJ mol- and AS = 223 J mol-1 K-1. What is AG for this…
A:
Q: Determine the EA for the rate-determining step (step 1) for this reaction coordinate diagram, in kJ…
A:
Q: I need help on my study problems. I was wondering if you could help with 6. Thanks!
A: The time needed for the decaying process of half of the original quantity of reacting species is…
Q: If a mixture was prepared by diluting 7.0 mL of the Blue#1 solution with water to a final volume of…
A: you have to use equation M1V1 = M2V2 15M(7)= M2 (12ml) and., M2 = 8.75 M b = 0.1044 k= - 0.0351
Q: 80 20 5 Determine the EA for the rate-determining step (step 1) for this reaction coordinate…
A: Minimum energy is required for reactant species to form products is known as activation energy.
Q: The rate constant for the reaction at 25.0 °C is 6.02 × 10^7 M -1 s -1. At 65,000 ft in the…
A:
Q: The reaction: 2 clO2 (aq) → ClO3- (ag) + C1O2¯ (ag) + H2O studied with the following experimental…
A:
Q: 2. For the reaction we studied: 21 + S2Og- 2 + 2 SO AErxn AHrxn = -322 kJ %3D A. Use this value and…
A: The explanation is given below-
Q: Given the following data, determine the rate law and calculate K Experiment…
A: Rate of reaction represents the change of concentration of a reactant or a product with respect to…
Q: mL of mL of 0.30 M [HCI] initial [Na,S,0,] initial [HCI] final [Na,S,0,1 final Reaction time (sec)…
A: The reaction rate is the rate of change of concentration with respect to the time and mathematically…
Q: 2. For the reaction we studied: 2 1- + S2Og²- –> I2 + 2 SO42- AErxn AHrxn = -322 kJ/mol A. Use this…
A: When the reactants are converted to products, some bonds are broken and some are formed. This causes…
Q: Table 2: Molarity of H2O2 and KI and Reaction Rate Trial H2O2 Concentration, M KI Concentration,…
A: Trial 1 slope = 14.47 sec/ml 1/slope = 0.069 ml/sec Trial 2 slope = 24.16 sec/ml 1/slope = 0.041…
Q: A proposed mechanism is shown below: (CH3)3CB1(aq) → (CH3)3C*(aq) + Br (aq) slow (CH3);C*(aq) +…
A: Since you have posted multiple questions, we are entitled to answer the first only.
Q: Bb Microso x I Bb Emailin x Bb CHEM 2 X Bb CHEM 2 X с C Expert X b My Que x + Content x Take Te X…
A:
Q: B. Reaction Rate and Concentration Concentration of H2C2O4 (M) | Time for permanganate ion to…
A:
Q: 2]o (M) [OH-]o (M) Initial Rate (M/s) 1 0.0500 0.100 5.75 x 10-2 2 0.100 0.100 2.30…
A:
Q: The equilibrium NH3(aq) + H20(1) →NH4*(aq) + OH¯(aq) at 25 °C is subjected to a temperature jump…
A:
Q: Q1) consider the following reaction: 2Xy X2 + Y2 The following data were obtained for this reaction…
A: First we have to find order of reaction. We use hit trial method. We find Rate constant, if two Rate…
Q: The value of AS° for the catalytic hydrogenation of acetylene to ethene, C2H2 (g) + H2 (g) → C2H4…
A: C) -112.0 J/mol.K Given reaction: C2H2 (g) + H2 (g) ----> C2H4 (g) The standard enthalpy…
Q: The equilibrium NH3(aq) + H20(1) -NH4*(aq) + OH (aq) at 25 *C is subjected to a temperature jump…
A:
Q: Calculate AHrn for the reaction: CeH12O6 (9 + O2(g → COz(0 + 6 H20 (0) AH: = -1273.3 kJ/mol AH, = 0…
A: Answer: Enthalpy of the reaction will be equal to the difference in enthalpy of formation of…
Q: A certain reaction Bn+ is getting converted to B(n+4)+ in solution. The rate constant of this…
A: The above question is solve by First order reaction
Q: Part A The reactant concontraion in A zeno-order ction was 500x10 Mater 120 s and 1.00x10 Mator 30s.…
A:
Q: Mixture [Acetone], [H.], [IL]o Rate = avg. time A1 .8 .2 .001 4.17*10^-6 A2 1.6 .2 .001 8.5*10^-6 АЗ…
A: Given: Rate=k[Acetone]m[I2]n[H+]p Now,…
Q: Determine the EA for the rate-determining step (step 1) for this reaction coordinate diagram, in kJ.…
A:
Q: N O,→2NDE * ½ Oz is . 2 Ng Og = NOg t NO, 3. beo NOtO,t NOz NO,+ NO3 NO t NOg 2. ky >2ND2 Appy…
A: Rate of reaction represents the change of concentration of a reactant or a product with respect to…
Q: Determine AH" and AS" for the second order reaction Br2(g) +12g) - 21Brg) which has a rate constant…
A: Given, rate constant k =8.56 X 10-5 M-1s-1Temperature, T = 50.0 oC =273 + 50 = 323 KActivation…
Q: 8)The following data were obtained from A + B = P+Qby brawlt Expt. | Initial Concentration 1.35 4…
A:
Q: Using the experimental data provided, determine the Activation Energy (in kJ/mol) of the following…
A: We are given the experimental value of the rate constant at two different temperatures, and we have…
Q: Determine the EA for the rate-determining step (step 1) for this reaction coordinate diagram, in kJ.…
A:
Q: DATA AND CALCULATIONS Rate constant, k (54) Trial Temperature (*C) 1 25 0.005144 20 0.004020 3 15…
A: The Arrhenius equation draws a relationship between the rate constant of a reaction, its activation…
Q: Given reaction rate date for: 2NO(g) + Br2(g) → 2NOBr(g) Trial [NO] (M) [Br2] (M) 1 0.20 0.020 2…
A: Given , Experimental results for rate of reaction at different concentrations. To find:- Rate law =…
Q: 800 705 kJ 600 A 497 kJ 200 175 kJ 100 Reaction Progress Created by E. Lee for Virtual Virginia…
A:
Q: Determine the Ecell and ΔG for the reaction of 2AgNO3 (aq, 1.0M) + Sn(s) ---> Sn(NO3)2(aq, 0.25M)…
A:
Q: Using the data in the table, determine the rate constant of the Trial [A] (M) [B] (M) Rate (M/s)…
A: Consider the reaction: A + 2B → C + D Let the order of the reaction with respect to A be α and the…
Q: Calculate the [KI] for Run A3 in Item 3 of Part I in your report pages. You will need the volumes on…
A: Given: Concentration of KI in stock solution = 0.20 M Volume of KI stock solution taken = 20.0 mL.…
Q: 1. Draw the following potentialenergy diagrams and fill in the missing values. Potential energy of…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: ep 1. H₂O₂ +J H₂O + 10 slow ep 2. 10 + H₂Qz H₂O + 0₂ + I fast The reaction coordinate diagram for…
A:
Q: Mass of Cu(NO,);.3H,O 2.3366 Volume of Deionized H,0 57.0 Concentration of Cu²* Solution Moles Cu?+…
A: Given:
Q: Using the following data to calculate Ksp for PbSO4. ε° PbO2 + 4H+ + SO42- + 2e-→ PbSO4(s) +…
A: Given: PbO2 + 4H+ + SO42- + 2e-→ PbSO4(s) + 2H2O E°= +1.69V PbO2 + 4H+ + 2e-→ Pb2+ + 2H2O E°= +1.46V
Q: Order with respect to KI:
A: We have been asked to find Order with respect to H2O2: Order with respect to KI:
Q: 2. Consider the kinetic data of the reaction of P + 2 Q + 22+ - PQ2 + Z2*and answer the succeeding…
A: The rate law of the reaction is: Rate = k * [P]2*[Q] where k = rate constant #D: In order to find…
Q: 0.29 M 0.40 M 14.08 2 0.29 M 0.20M 25 3 0.023 M 0.40 M 20 Order with respect to H2O2:…
A: Given : Rate constants of different - different trials. To find : Explanation for the rate…
Q: Reaction: A + B+C → ABC [A] [B] Rate Trial 1 0.5 M 1.5 M 1.0 M 0.10 M/s Trial 2 0.5 M 1.5 M 2.0 M…
A: Solution : The rate law shows the relation between the rate of reaction and the concentration…
Q: Determine AH" and AS" for the second order reaction Brag + I2o- 21Brg which has a rate constant of…
A: Activation energy can be written as the sum of the change in enthalpy and RT.…
Q: Calculate the AS°rxn of the following reaction at 223°C and standard pressure. 1st attempt O See…
A:
Q: Given reaction rate date for: 2NO(g) + Br₂(g) Trial [NO] (M) 1 0.20 2 0.20 3 0.30 2NOBr(g) [Br2] (M)…
A:
Q: For the reaction 2Cog) + Ozls) → 2CO13). Reaction Trial Initial conc. CO M Initial conc. O2 M…
A:
Q: (a) Can an intermediate appear as a reactant in the firststep of a reaction mechanism? (b) On a…
A: (a) The reactant dissociates or associates in the first step of the reaction and leads to the…
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 12 images

- Prof. Neiman and Prof. James were first to discovered in 1973 that chlorofluorocarbons (CFCs) were depleting the Earth’sozone layer when released into the atmosphere. Once they reach the stratosphere, Clis released from the CFCs molecules by interaction with UV light. Free Cl atoms areable to react with ozone in a catalytic cycle that converts O3into the more stable O2.It is estimated that a single Cl atom is able to react with∼100000 O3molecules.Although CFCs production was banned in 1996, there are still a substantial numberof motor vehicle air conditioners (MVACs) that use CFC-12 (CF2Cl2) as refrigerant.The average CFC-12 emission rate from operating MVACs has been estimated tobe 59.5 mg per hour per vehicle (Zhang et al.Environ. Sci. Technol. Lett.2017).How much chlorine, in kg, is added to the atmosphere in a year due to 100 millionMVACs using CFC-12 as refrigerant?Reaction of interest : S2O82-(aq) + 3I- (aq)→ 2SO42-(aq) + I3-(aq). rate= k[S2O82-]1[I-]1.need help with this ASAP pls & thank you! (it's asking for you to fill in the blank boxes)
- The data below was collected from three trials of the following reaction: 2ClO2 (aq) + 2OH- (aq) --> ClO3- (aq) + ClO2- (g) + H2O (l) [ClO2]o (M) [OH-]o (M) Initial Rate (M/s) 1 0.0500 0.100 5.75 x 10-2 2 0.100 0.100 2.30 x 10-1 3 0.100 0.0500 1.15 x 10-1 If the initial concentrations were as follows, [ClO2]o= 0.25 M and [OH- ]o = 0.095 M, what would the initial rate be?Reducing NO Emissions Adding NH3 to the stack gases at an electric power generating plant can reduceNOx emissions. This selective noncatalytic reduction (SNR) process depends on the reaction between NH3 (an odd-electron compound) and NO.$$4NH3(g)+6NO(g)5N2(g)+6H2O(g)The following kinetic data were collected at 1200 K. Experiment [NH3] (M) [NO] (M) Rate (M/s) 1 1.00x10-5 1.00x10-5 0.120 2 2.00x10-5 1.00x10-5 0.240 3 2.00x10-5 1.50x10-5 0.360 4 2.50x10-5 1.50x10-5 0.450 What is the rate-law expression for the reaction? Do not add multiplication symbols to your answer. $$Rate=Instantaneous rates for the reaction of hydroxide ion with Cv+ can be determined from the slope of the curve in Figure 11.3 at various concentrations. They are (1) At 4.0 105 mol/L, rate = 12.3 107 mol L1 s1 (2) At 3.0 105 mol/L, rate = 9.25 107 mol L1 s1 (3) At 2.0 105 mol/L, rate = 6.16 107 mol L1 s1 (4) At 1.5 105 mol/L, rate = 4.60 107 mol L1 s1 (5) At 1.0 105 mol/L, rate = 3.09 107 mol L1 s1 (a) What is the relationship between the rates in (1) and (3)? Between (2) and (4)? Between (3) and (5)? (b) What is the relationship between the concentrations in each of these cases? (c) Is the rate of the reaction proportional to the concentration of Cv+? Explain your answer.
- Which of the following is a spontaneous reaction.? a. Rxn with ΔH =- 10Kj/mol ΔS= -5J/mol T= 300K b. NaCl +H20 -> NaOH + HCl 25C c. H20(l) -> H2O(s) Temp: 25C d. Dissolution of 100g of solid sugar in 100 mL ice tea. Consider following reaction: HgO (s) -> Hg(l) + ½ O2 (g) Delta H = +90.7 kj/mol. What quantity of heat in kj/mol is required to produce one mole HgO? Write your answer without units. Given the following data 2ClF(g) + O2(g) --> Cl2O(g) + F2O (g) Delta H= 167.4 kJ I 2ClF3(g) + 2O2(g) --> Cl2O(g) + 3F2 O (g) Delta H= 341.4 kJ II 2 F2(g) + O2(g) ---> 2F2O (g) Delta H= -43.4 kJ III Calculate the delta H in kJ for below reaction: ClF(g) + F2(g) ---> ClF3(g)Pls show eork, will rate, D,E,FPls solve this problem and I upvote. Do not reject if it's hard for you skip it so one of the expert can do it. Thank you. I pray for your wellbeing
- UCI Chemistry researchers, Prof. F. Sherwood Rowland and Dr. Mario Molina werefirst to discovered in 1973 that chlorofluorocarbons (CFCs) were depleting the Earth’sozone layer when released into the atmosphere. Once they reach the stratosphere, Clis released from the CFCs molecules by interaction with UV light. Free Cl atoms areable to react with ozone in a catalytic cycle that converts O3into the more stable O2.It is estimated that a single Cl atom is able to react with∼100000 O3molecules.Although CFCs production was banned in 1996, there are still a substantial numberof motor vehicle air conditioners (MVACs) that use CFC-12 (CF2Cl2) as refrigerant.The average CFC-12 emission rate from operating MVACs has been estimated tobe 59.5 mg per hour per vehicle (Zhang et al.Environ. Sci. Technol. Lett.2017).How much chlorine, in kg, is added to the atmosphere in a year due to 100 millionMVACs using CFC-12 as refrigerant?The peroxydisulfate ion (S2O8-2) reacts with the iodide ion in aqueous solution via the reaction: S2O82-(aq) + 3I- → 2SO4(aq)+ I3-(aq). An aqueous solution containing 0.050 M of S2O8-2 ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. Time (s) 0.00 400.0 800.0 1200.0 1600.0 [I‑] (M) 0.072 0.057 0.046 0.037 0.029 Determine the concentration of S2O82- remaining at 400 s in M. Determine the concentration of S2O82- remaining at 800 s in M. Determine the concentration of S2O82- remaining at 1600 s in M.The peroxydisulfate ion (S2O8-2) reacts with the iodide ion in aqueous solution via the reaction: S2O82-(aq) + 3I- → 2SO4(aq)+ I3-(aq). An aqueous solution containing 0.050 M of S2O8-2 ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. Time (s) 0.00 400.0 800.0 1200.0 1600.0 [I‑] (M) 0.072 0.057 0.046 0.037 0.029 Determine the average rate of disappearance of I- between 400.0 s and 800.0 s in M/s. Determine the average rate of disappearance of I- in the initial 400.0 s in M/s. Determine the average rate of disappearance of I- between 1200.0 s and 1600.0 s in M/s. Determine the concentration of S2O82- remaining at 400 s in M. Determine the concentration of S2O82- remaining at 800 s in M. Determine the concentration of S2O82- remaining at 1600 s in M.



