Homework 8 1. Calculate, in the standard state: (1) What is the average translational kinetic energy of the oxygen molecules and the hydrogen molecules? (2) How many molecules are there in 1 m³ gas? 2. Supposing there is one mole oxygen at 300K, find (1) the translational kinetic energy; (2) the rotational kinetic energy for the gas. How about the change of the total kinetic energy when AT=IK? 3. What is the change in internal energy as the temperature increases 1K? (1) For 1 mol of monatomic molecules, 1 mol diatomic molecules and Imol polyatomic molecules respectively. (2) Answer the same question for 1g of N₂ and 1g of H₂.
Homework 8 1. Calculate, in the standard state: (1) What is the average translational kinetic energy of the oxygen molecules and the hydrogen molecules? (2) How many molecules are there in 1 m³ gas? 2. Supposing there is one mole oxygen at 300K, find (1) the translational kinetic energy; (2) the rotational kinetic energy for the gas. How about the change of the total kinetic energy when AT=IK? 3. What is the change in internal energy as the temperature increases 1K? (1) For 1 mol of monatomic molecules, 1 mol diatomic molecules and Imol polyatomic molecules respectively. (2) Answer the same question for 1g of N₂ and 1g of H₂.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 81AP
Related questions
Question

Transcribed Image Text:08:41
<
46...
Homework 8-Kinetic theory of Gases.pdf
Homework 8
1. Calculate, in the standard state:
(1) What is the average translational kinetic energy of the oxygen
molecules and the hydrogen molecules?
(2) How many molecules are there in 1 m³ gas?
2. Supposing there is one mole oxygen at 300K, find (1) the translational
kinetic energy; (2) the rotational kinetic energy for the gas. How about
the change of the total netic energy when AT-IK?
3. What is the change in internal energy as the temperature increases 1K?
(1) For 1 mol of monatomic molecules, Imol diatomic molecules and
1mol polyatomic molecules respectively. (2) Answer the same
question for 1g of N₂ and 1g of H₂.
85%
←
Transcribed Image Text:13:00
<
ا... 46
of
Homework 9-Foundamentals
thermodynamics(2).pdf
Homework 9
1. A monatomic ideal gas is contained in a cylinder closed with a
movable piston. The initial pressure is 8 atm. and the initial volume is
0.5L. The gas is heated first at constant pressure until the volume
becomes to 1.5L, then expands isothermally until the volume is
doubled, finally cooled down at constant volume until the pressure
drops to 1 atm. (1) How much work is done by the gas in the three
processes? (2) What is the change in internal energy? (3) How much
heat is supplied to system?
2. 2 mol of Helium (assume an ideal gas) expanded adiabatically from
an initial state (T; =300K) until the volume is doubled. (1) Calculate
the work done by the gas; (2) What would be the answer if the gas had
expanded isothermally from the same initial state to the same final
volume?
Pt
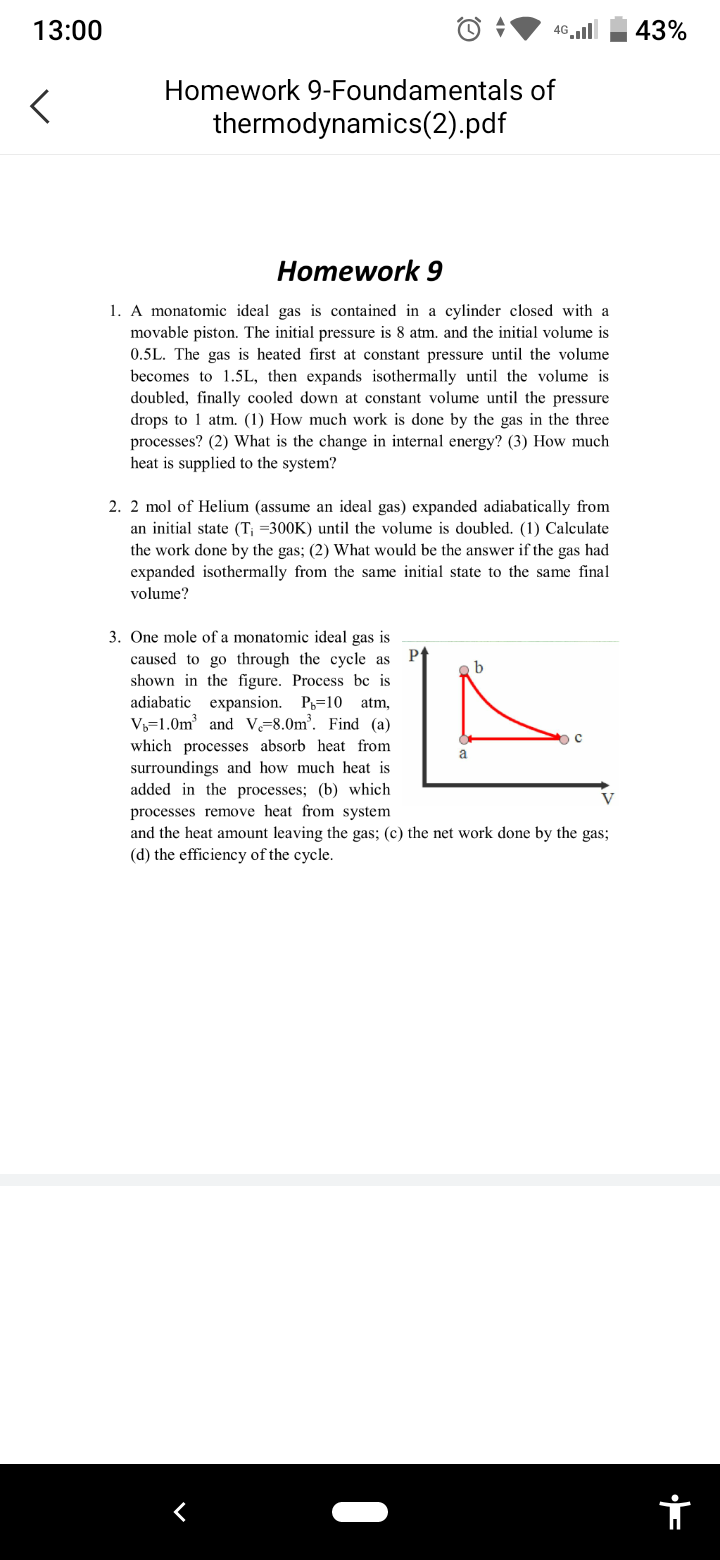
3. One mole of a monatomic ideal gas is
caused to go through the cycle as
shown in the figure. Process be is
adiabatic expansion. P-10 atm,
V-1.0m³ and V-8.0m³. Find (a)
which processes absorb heat from
surroundings and how much heat is
added in the processes; (b) which
a
V
processes remove heat from system
and the heat amount leaving the gas; (c) the net work done by the gas;
(d) the efficiency of the cycle.
43%
←
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning