Homework: Find the molarity of solution of HCl that has a density of 1.19 gimiL and contains 37.0 (w/w %). How much volume is needed to prepare 100ml of 6.0M HCI from concentrated solution?
Homework: Find the molarity of solution of HCl that has a density of 1.19 gimiL and contains 37.0 (w/w %). How much volume is needed to prepare 100ml of 6.0M HCI from concentrated solution?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 23QAP
Related questions
Question
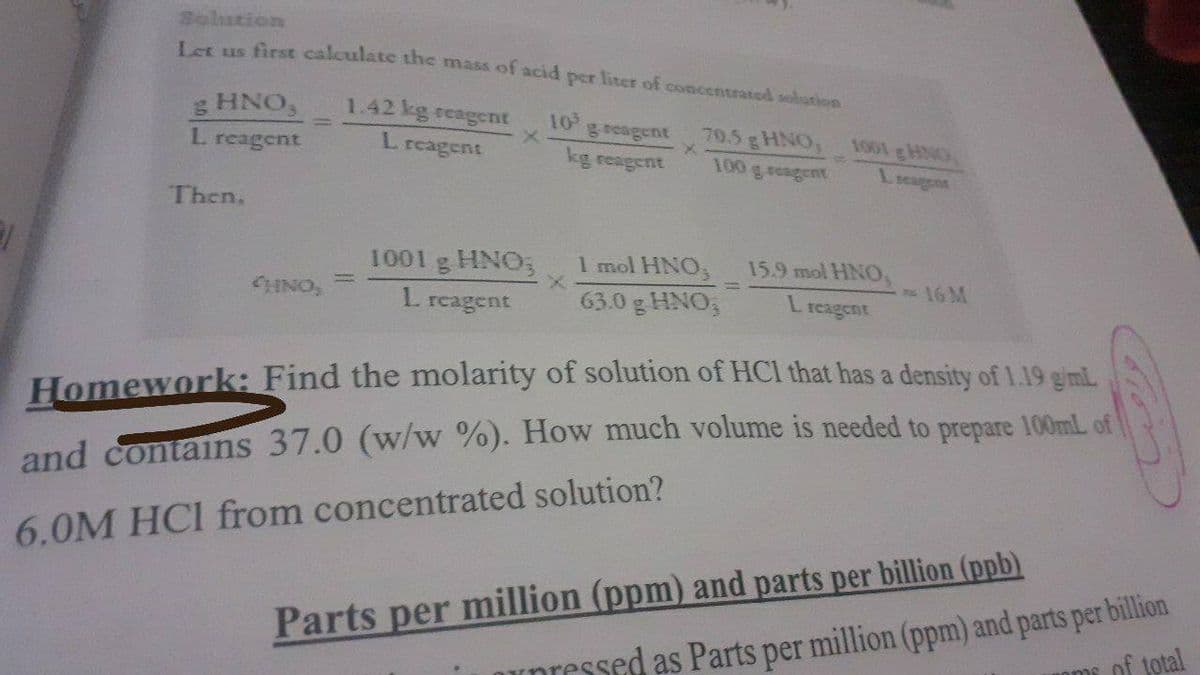
Transcribed Image Text:Solution
Us first calculate the mass of acid per liter of concentrated solution
g HNO,
L reagent
1.42 kg reagent
10
geeagent
70.5 gHNO, 1001 gHNG
100 g reagent
L reagent
kg reagent
Lieaggnt
Then,
1001 g HNO;
1 mol HNO,
15.9 mol HNO,
CHINO,
16 M
L reagent
63.0 g HNO,
L.
reagent
Homework: Find the molarity of solution of HCl that has a density of 1.19 giml
and contains 37.0 (w/w %). How much volume is needed to prepare 100mlL of
6.0M HCI from concentrated solution?
upressed as Parts per million (ppm) and parts per billion
oms of total
Parts per million (ppm) and parts per billion (ppb)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
