Hydrogen fluoride (HF) reacts with dichlorodifluoromethane (D) to produce tetrafluoromethane (T) and HCl according to the following reaction: Liquid D is fed with HF to a reactor where the single-pass conversion of D in the reactor is 70.0%. The reactor effluent enters a distillation column where the bottoms contains 95.0% of the unreacted D and the overhead contains all of the products, the unreacted HF, and the balance of the unreacted D. The bottoms are recycled back to mix with the fresh feed that enters the reactor. If the fresh feed contains 10.0 mol/s of D and 25.0% excess HF, how much D enters the reactor in one minute (kg)? MW D = 120.9.
Hydrogen fluoride (HF) reacts with dichlorodifluoromethane (D) to produce tetrafluoromethane
(T) and HCl according to the following reaction:
Liquid D is fed with HF to a reactor where the single-pass conversion of D in the reactor is 70.0%.
The reactor effluent enters a distillation column where the bottoms contains 95.0% of the
unreacted D and the overhead contains all of the products, the unreacted HF, and the balance of
the unreacted D. The bottoms are recycled back to mix with the fresh feed that enters the reactor. If
the fresh feed contains 10.0 mol/s of D and 25.0% excess HF, how much D enters the reactor in
one minute (kg)? MW D = 120.9.
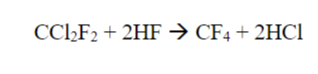
Tetrafluoromethane and HCl are produced by the reaction of dichlorodilfluoromethane and hydrogen fluoride.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

how did you know the CCl2F2 in the reactor effluent was = (1-.7)*(10+R)?
And how were you able to solve for R with the given information?
how did you know to times the 20 mol of HF by 1.25?








